CXCR4 promotes differentiation of oligodendrocyte progenitors and remyelination - PubMed (original) (raw)
CXCR4 promotes differentiation of oligodendrocyte progenitors and remyelination
Jigisha R Patel et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Multiple sclerosis is a neurodegenerative disease characterized by episodes of autoimmune attack of oligodendrocytes leading to demyelination and progressive functional deficits. Because many patients exhibit functional recovery in between demyelinating episodes, understanding mechanisms responsible for repair of damaged myelin is critical for developing therapies that promote remyelination and prevent disease progression. The chemokine CXCL12 is a developmental molecule known to orchestrate the migration, proliferation, and differentiation of neuronal precursor cells within the developing CNS. Although studies suggest a role for CXCL12 in oligodendroglia ontogeny in vitro, no studies have investigated the role of CXCL12 in remyelination in vivo in the adult CNS. Using an experimental murine model of demyelination mediated by the copper chelator cuprizone, we evaluated the expression of CXCL12 and its receptor, CXCR4, within the demyelinating and remyelinating corpus callosum (CC). CXCL12 was significantly up-regulated within activated astrocytes and microglia in the CC during demyelination, as were numbers of CXCR4+NG2+ oligodendrocyte precursor cells (OPCs). Loss of CXCR4 signaling via either pharmacological blockade or in vivo RNA silencing led to decreased OPCs maturation and failure to remyelinate. These data indicate that CXCR4 activation, by promoting the differentiation of OPCs into oligodendrocytes, is critical for remyelination of the injured adult CNS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
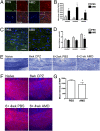
CXCL12 expression in the CC after CPZ exposure. (A) qRT-PCR analysis of CXCL12 expression in dissected whole CC derived from naive mice and from mice after 6 and 12 wk of CPZ ingestion. Each group represents three to five individual animals, *, P < 0.05. (B) Confocal IHC analysis of CC from naive mice and after 6 and 12 wk of CPZ ingestion. Images were stained for GFAP or CD11b (green, Upper and Lower, respectively), CXCL12 (red), and nuclei (blue). Higher power images of boxed areas in images from 6-wk time-point are shown. Representative images shown for three sections from three mice in two separate experiments. IC = isotype control. (Scale bars, 25 μm.) (C and D) Quantitation of GFAP+ (C) and CD11b+ (D) cells that express CXCL12 within rostral, central, and caudal sections of the CC in naive mice (white bars) and after 6 (gray bars) and 12 (black bars) wk of CPZ exposure; n = 6 images taken from three to five mice/group. *, P < 0.05 and **, P < 0.005.
Fig. 2.
CXCR4 expression in the CC after CPZ exposure. (A) QRT-PCR analysis of CXCR4 expression in dissected whole CC derived from naive mice and from mice after 6 and 12 wk of CPZ ingestion. Each group represents three to five individual animals, *, P < 0.05. (B) Confocal IHC analysis of CC from naive mice and after 6 and 12 wk of CPZ ingestion. Images were stained for NG2 (green), CXCL12 (red), and nuclei (blue). Higher power images of boxed area in image from 6-wk time-point are shown. Representative images shown for three sections from three mice in two separate experiments. IC = isotype control. (Scale bars, 25 μm.) (C) Quantitation of NG2+ cells that express CXCL12 within rostral, central, and caudal sections of the CC in naive mice (white bars) and after 6 (gray bars) and 12 (black bars) wk of CPZ exposure; n = 6 images taken from three to five mice/group. *, P < 0.05 and **, P < 0.005.
Fig. 3.
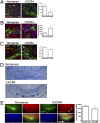
CXCR4 antagonism prevents maturation of OPCs and remyelination. (A) Confocal IHC analysis of CC from mice after 6 wk of CPZ ingestion then 2 wk of treatment with PBS (Left) or AMD3100 (Right, AMD). Sections were stained for CXCR4 (green), NG2 (red), and nuclei (blue). Note linear organization of NG2-negative, oligodendrocyte nuclei in PBS-treated specimen (arrowheads). (Inset) Isotype control. (Scale bar, 25 μm.) (B) Quantitation of CXCR4+NG2+ cells within rostral, central and caudal sections of the CC in naive mice (white bars) and after 6 (gray bars) and 12 (black bars) wk of CPZ exposure; n = 6 images taken from four mice/group. *, P < 0.05. (C) Sections from CC of PBS- (Left) or AMD3100- (Right, AMD) treated mice were stained for CNPase (green) and nuclei (blue). (Inset) Isotype control. (Scale bar, 25 μm.) (D) Quantitation of CNPase+ cells within rostral, central, and caudal sections of the CC in naive mice (white bars) and after 6 (gray bars) and 12 (black bars) wk of CPZ exposure; n = 6 images taken from four mice/group. *, P < 0.05. (E) LFB myelin staining of CC in mice at 0 and 6 wk of CPZ treatment and after 6 wk of CPZ treatment plus 2 wk of treatment with PBS or AMD3100 (AMD). (F) IHC analysis of MBP expression (red) within CC of naive mice (Upper Left) and in mice treated with 4 wk of PBS (Lower Left) or AMD3100 (Lower Right, AMD) after 6 wk of CPZ ingestion. Nuclei counterstained with DAPI (blue). (Scale bar, 50 μm.) (G) Quantitation of MBP expression within all regions of the CC in mice treated with 4 wk of PBS or AMD3100 (AMD) after 6 wk of CPZ ingestion; n = 6 images taken from four mice/group. *, P < 0.05.
Fig. 4.
CXCR4 activation differentially affects OPC proliferation within the SVZ and CC. (A) Confocal IHC analysis of rostral subventricular zones (SVZ) and CC from mice after 6 wk of CPZ ingestion plus 2 wk of treatment with PBS (Left) or AMD3100 (Right) with daily i.p. injection of BrDU. Images were stained for BrDU (green), NG2 (red), and nuclei (blue). Representative images shown from three sections from three individual mice per group. (Scale bars, 25 μm.) (B) Quantitation of BrDU+NG2+ cells within rostral, central, and caudal sections of the CC and SVZ of mice after 6 wk of CPZ ingestion and 2 wk of treatment with PBS (gray bars) or AMD3100 (black bars); n = 6 images taken from three mice/group, *, P < 0.05.
Fig. 5.
CXCR4 RNA silencing in glial cells in vitro. (A) Schematic of lentiviral construct used to silence CXCR4 expression. pLKO.1 plasmids carrying shRNAs (CXCR4 and nonsense) driven by the human U6 promoter and HIV packaging signal were reengineered to contain sequence encoding GFP with a polyA tail driven by the CMV promoter. CXCR4 shRNA and nonsense sequences are shown. (B) qPCR analysis of CXCR4 mRNA levels in primary astrocytes infected with CXCR4 shRNA lentivirus at multiplicity of infection (MOI) of 5, 10, and 20. Data are presented as the fold-change in CXCR4 mRNA levels normalized to those observed in astrocytes infected with nonsense shRNA lentivirus. (C) Confocal IHC analysis of GFP (green), CXCR4 (red), and NG2 (blue) expression by primary oligodendroglia infected with nonsense (Left) versus CXCR4 (Middle) shRNA lentiviruses at 20 MOIs reveals significant knock-down of CXCR4 as assessed by confocal IHC (Right); n = 5–6 images taken from three replicates/group, *, P < 0.05. (D) Low power images depicting stereotactic infection of CC with GFP-expressing lentiviruses expressing nonsense (Left) and CXCR4 (Right) shRNAs. Arrow = site of injection. (Scale bar, 50 μm.)
Fig. 6.
In vivo CXCR4 silencing prevents OPC maturation and remyelination. (A and B) Confocal IHC analysis of CC from mice after 6 wk of CPZ ingestion followed by stereotactic injection of lentivirus bearing shRNAs (Nonsense, Left, versus CXCR4, Right). Images depict GFP expression (green) with staining for CXCR4 (A), NG2 (B), and CNPase (C). (Scale bar, 20 μm.) Quantitation of CXCR4+ (A), NG2+ (B), and CNPase+ (C) cells within CC of mice injected with lentivirus expressing Nonsense and CXCR4 shRNAs after 6 wk of CPZ exposure (Far Right); n = 6 images taken from three to five mice/group. *, P < 0.05. (D) LFB-stained sections of CC from mice injected with lentivirus expressing Nonsense (Upper) and CXCR4 (Lower) shRNAs after 6 wk of CPZ exposure. (A_–_D) Representative images shown for three sections from three to five mice in two separate experiments. Note linear area without remyelination in mouse injected with CXCR4 shRNA lentivirus (arrowheads). (E) IHC analysis of MBP expression (red) within CC of mice injected with lentivirus expressing GFP (green) plus Nonsense (Left) and CXCR4 (Right) shRNAs after 6 wk of CPZ exposure. Note area of demyelination in CC of mouse with CXCR4 RNA silencing that correspond to sites of GFP staining (arrowheads). Nuclei counterstained with DAPI (blue). Quantitation of MBP staining in areas of GFP expression was performed on two to three sections/mouse in three to five mice/group in two separate experiments, *, P < 0.05 (Far Right).
Comment in
- Repair: Chemokines show the way.
Welberg L. Welberg L. Nat Rev Neurosci. 2010 Aug;11(8):536-7. doi: 10.1038/nrn2887. Nat Rev Neurosci. 2010. PMID: 20672429 No abstract available.
Similar articles
- Astrocyte TNFR2 is required for CXCL12-mediated regulation of oligodendrocyte progenitor proliferation and differentiation within the adult CNS.
Patel JR, Williams JL, Muccigrosso MM, Liu L, Sun T, Rubin JB, Klein RS. Patel JR, et al. Acta Neuropathol. 2012 Dec;124(6):847-60. doi: 10.1007/s00401-012-1034-0. Epub 2012 Aug 30. Acta Neuropathol. 2012. PMID: 22933014 Free PMC article. - Migration of engrafted neural stem cells is mediated by CXCL12 signaling through CXCR4 in a viral model of multiple sclerosis.
Carbajal KS, Schaumburg C, Strieter R, Kane J, Lane TE. Carbajal KS, et al. Proc Natl Acad Sci U S A. 2010 Jun 15;107(24):11068-73. doi: 10.1073/pnas.1006375107. Epub 2010 Jun 1. Proc Natl Acad Sci U S A. 2010. PMID: 20534452 Free PMC article. - Targeting CXCR7/ACKR3 as a therapeutic strategy to promote remyelination in the adult central nervous system.
Williams JL, Patel JR, Daniels BP, Klein RS. Williams JL, et al. J Exp Med. 2014 May 5;211(5):791-9. doi: 10.1084/jem.20131224. Epub 2014 Apr 14. J Exp Med. 2014. PMID: 24733828 Free PMC article. - The role of oligodendrocytes and oligodendrocyte progenitors in CNS remyelination.
Keirstead HS, Blakemore WF. Keirstead HS, et al. Adv Exp Med Biol. 1999;468:183-97. doi: 10.1007/978-1-4615-4685-6_15. Adv Exp Med Biol. 1999. PMID: 10635029 Review. - Mechanisms of remyelination: recent insight from experimental models.
Tanaka T, Yoshida S. Tanaka T, et al. Biomol Concepts. 2014 Aug;5(4):289-98. doi: 10.1515/bmc-2014-0015. Biomol Concepts. 2014. PMID: 25372760 Review.
Cited by
- Promoting remyelination in multiple sclerosis-recent advances.
Münzel EJ, Williams A. Münzel EJ, et al. Drugs. 2013 Dec;73(18):2017-29. doi: 10.1007/s40265-013-0146-8. Drugs. 2013. PMID: 24242317 Free PMC article. Review. - Glial PAMPering and DAMPening of Adult Hippocampal Neurogenesis.
Parkitny L, Maletic-Savatic M. Parkitny L, et al. Brain Sci. 2021 Sep 29;11(10):1299. doi: 10.3390/brainsci11101299. Brain Sci. 2021. PMID: 34679362 Free PMC article. Review. - The role of CXCR4 signaling in the migration of transplanted oligodendrocyte progenitors into the cerebral white matter.
Banisadr G, Frederick TJ, Freitag C, Ren D, Jung H, Miller SD, Miller RJ. Banisadr G, et al. Neurobiol Dis. 2011 Oct;44(1):19-27. doi: 10.1016/j.nbd.2011.05.019. Epub 2011 Jun 6. Neurobiol Dis. 2011. PMID: 21684336 Free PMC article. - Attempts to Overcome Remyelination Failure: Toward Opening New Therapeutic Avenues for Multiple Sclerosis.
Motavaf M, Sadeghizadeh M, Javan M. Motavaf M, et al. Cell Mol Neurobiol. 2017 Nov;37(8):1335-1348. doi: 10.1007/s10571-017-0472-6. Epub 2017 Feb 21. Cell Mol Neurobiol. 2017. PMID: 28224237 Free PMC article. Review. - Distant origin of glioblastoma recurrence: neural stem cells in the subventricular zone serve as a source of tumor reconstruction after primary resection.
Li X, Kim HJ, Yoo J, Lee Y, Nam CH, Park J, Lee ST, Kim TM, Choi SH, Won JK, Park SH, Ju YS, Park JB, Kim SH, Chang JH, Wu HG, Park CK, Lee JH, Kang SG, Lee JH. Li X, et al. Mol Cancer. 2025 Mar 4;24(1):64. doi: 10.1186/s12943-025-02273-2. Mol Cancer. 2025. PMID: 40033380 Free PMC article.
References
- Weiner HL. The challenge of multiple sclerosis: how do we cure a chronic heterogeneous disease? Ann Neurol. 2009;65:239–248. - PubMed
- Blakemore WF, Keirstead HS. The origin of remyelinating cells in the central nervous system. J Neuroimmunol. 1999;98:69–76. - PubMed
- Wilson HC, Scolding NJ, Raine CS. Co-expression of PDGF alpha receptor and NG2 by oligodendrocyte precursors in human CNS and multiple sclerosis lesions. J Neuroimmunol. 2006;176:162–173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials