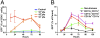Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus - PubMed (original) (raw)
Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus
Balaji Manicassamy et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Influenza A virus is being extensively studied because of its major impact on human and animal health. However, the dynamics of influenza virus infection and the cell types infected in vivo are poorly understood. These characteristics are challenging to determine, partly because there is no efficient replication-competent virus expressing an easily traceable reporter gene. Here, we report the generation of a recombinant influenza virus carrying a GFP reporter gene in the NS segment (NS1-GFP virus). Although attenuated when compared with wild-type virus, the NS1-GFP virus replicates efficiently in murine lungs and shows pathogenicity in mice. Using whole-organ imaging and flow cytometry, we have tracked the dynamics of influenza virus infection progression in mice. Imaging of murine lungs shows that infection starts in the respiratory tract in areas close to large conducting airways and later spreads to deeper sections of the lungs. In addition to epithelial cells, we found GFP-positive antigen-presenting cells, such as CD11b(+)CD11c(-), CD11b(-)CD11c(+), and CD11b(+)CD11c(+), as early as 24 h after intranasal infection. In addition, a significant proportion of NK and B cells were GFP positive, suggesting active infection of these cells. We next tested the effects of the influenza virus inhibitors oseltamivir and amantadine on the kinetics of in vivo infection progression. Treatment with oseltamivir dramatically reduced influenza infection in all cell types, whereas, surprisingly, amantadine treatment more efficiently blocked infection in B and NK cells. Our results demonstrate high levels of immune cells harboring influenza virus antigen during viral infection and cell-type-specific effects upon treatment with antiviral agents, opening additional avenues of research in the influenza virus field.
Conflict of interest statement
Conflict of interest statement: Mount Sinai School of Medicine has filed a patent application covering the use of recombinant influenza viruses expressing a reporter gene.
Figures
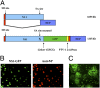
Fig. 1.
Generation of recombinant influenza virus carrying a GFP reporter. (A) Schematic representation of NS segment of WT PR8 virus and NS1-GFP virus. Splice acceptor site in NS was mutated to prevent mRNA splicing (SD-splice donor site, SA- splice acceptor site). Common regions present in both NS1 (light blue) and NEP (dark blue) are shown in red. NS1was fused to GFP (green) via a GSGG linker, followed by PTV-1 2A autproteolytic cleavage site (yellow) and NEP ORF (red-blue). (B) A549 cells were infected with recombinant PR8 virus carrying NS1-GFP. At 10 hpi, cells were fixed and stained for NP. NP staining is shown in red and NS1-GFP is shown in green. (C) Fluorescent micrographs of NS1-GFP virus plaques taken at 20× magnification.
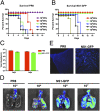
Fig. 2.
In vivo characterization of NS1-GFP virus. (A and B) Comparison of survival of PR8 and NS1-GFP virus–infected mice. BALB/c mice were intranasally inoculated with indicated doses of PR8 or NS1-GFP virus. Survival was monitored daily. (C) Viral titers in lungs of mice infected with WT PR8 virus and with NS1-GFP viruses. (D) Mice were intranasally inoculated with either PR8 or NS1-GFP virus at indicated doses. Lungs were excised on day 4 postinfection, and fluorescence from infected lungs was imaged using an IVIS-200 imaging system (Xenogen). (E) Fluorescent micrographs of mice lung cryosections (10× magnification).
Fig. 3.
Dynamics of influenza virus infection in lungs. (A) Kinetics of epithelial cell infection. BALB/c mice were intranasally inoculated with NS1-GFP virus at indicated doses, and lung homogenates were analyzed for GFP expression in nonhematopoietic cells (CD45−) using a BD LSR II flow cytometer. (B) Comparison of kinetics of hematopoietic and nonhematopoietic cell infection in murine lungs. BALB/c mice were intranasally inoculated with 106 pfu NS1-GFP virus and analyzed for GFP expression in cells types differentially expressing CD11b and CD11c. Each data point represents the average from at least three mice.
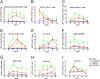
Fig. 4.
Oseltamivir and amantadine treatment significantly reduces NS1-GFP virus infection. Mice infected with NS1-GFP virus either were left untreated or were treated with oseltamivir (50 mg/kg) or amantadine (40 mg/kg), starting 1 h postinfection. Kinetics of infection progression in different cell types were analyzed using a BD LSR II flow cytometer. (A–G) Kinetics of GFP expression in different cell types as indicated in treated and untreated groups.
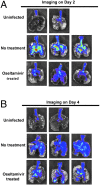
Fig. 5.
Oseltamivir treatment restricts influenza virus infection to localized areas. Ex vivo imaging of mice lungs on day 2 (A) and day 4 (B) postinfection. BALB/c mice infected with NS1-GFP virus (106 pfu) either were left untreated or were treated daily once with oseltamivir (50 mg/kg). Lungs of mice were excised at indicated time and imaged using the IVIS-200 system (Xenogen).
Similar articles
- Recombinant Influenza A Viruses Expressing Reporter Genes from the Viral NS Segment.
Martinez-Sobrido L, Nogales A. Martinez-Sobrido L, et al. Int J Mol Sci. 2024 Oct 1;25(19):10584. doi: 10.3390/ijms251910584. Int J Mol Sci. 2024. PMID: 39408912 Free PMC article. Review. - A GFP expressing influenza A virus to report in vivo tropism and protection by a matrix protein 2 ectodomain-specific monoclonal antibody.
De Baets S, Verhelst J, Van den Hoecke S, Smet A, Schotsaert M, Job ER, Roose K, Schepens B, Fiers W, Saelens X. De Baets S, et al. PLoS One. 2015 Mar 27;10(3):e0121491. doi: 10.1371/journal.pone.0121491. eCollection 2015. PLoS One. 2015. PMID: 25816132 Free PMC article. - A Novel Fluorescent and Bioluminescent Bireporter Influenza A Virus To Evaluate Viral Infections.
Nogales A, Ávila-Pérez G, Rangel-Moreno J, Chiem K, DeDiego ML, Martínez-Sobrido L. Nogales A, et al. J Virol. 2019 May 1;93(10):e00032-19. doi: 10.1128/JVI.00032-19. Print 2019 May 15. J Virol. 2019. PMID: 30867298 Free PMC article. - Directed Evolution of an Influenza Reporter Virus To Restore Replication and Virulence and Enhance Noninvasive Bioluminescence Imaging in Mice.
Cai H, Liu M, Russell CJ. Cai H, et al. J Virol. 2018 Jul 31;92(16):e00593-18. doi: 10.1128/JVI.00593-18. Print 2018 Aug 15. J Virol. 2018. PMID: 29899096 Free PMC article. - Infection of mice with influenza A/WSN/33 (H1N1) virus alters alveolar type II cell phenotype.
Hofer CC, Woods PS, Davis IC. Hofer CC, et al. Am J Physiol Lung Cell Mol Physiol. 2015 Apr 1;308(7):L628-38. doi: 10.1152/ajplung.00373.2014. Epub 2015 Jan 16. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 25595651 Free PMC article.
Cited by
- Recombinant Influenza A Viruses Expressing Reporter Genes from the Viral NS Segment.
Martinez-Sobrido L, Nogales A. Martinez-Sobrido L, et al. Int J Mol Sci. 2024 Oct 1;25(19):10584. doi: 10.3390/ijms251910584. Int J Mol Sci. 2024. PMID: 39408912 Free PMC article. Review. - Screening for anti-influenza virus compounds from traditional Mongolian medicine by GFP-based reporter virus.
Nie MS, Li XH, Zhang S, Zeng DD, Cai YR, Peng DX, Jiang T, Shi JP, Li J. Nie MS, et al. Front Cell Infect Microbiol. 2024 Jul 12;14:1431979. doi: 10.3389/fcimb.2024.1431979. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39071166 Free PMC article. - Primary nasal influenza infection rewires tissue-scale memory response dynamics.
Kazer SW, Match CM, Langan EM, Messou MA, LaSalle TJ, O'Leary E, Marbourg J, Naughton K, von Andrian UH, Ordovas-Montanes J. Kazer SW, et al. Immunity. 2024 Aug 13;57(8):1955-1974.e8. doi: 10.1016/j.immuni.2024.06.005. Epub 2024 Jul 3. Immunity. 2024. PMID: 38964332 - Novel transcription and replication-competent virus-like particles system modelling the Nipah virus life cycle.
Wang Y, Fan L, Ye P, Wang Z, Liang C, Liu Q, Yang X, Long Z, Shi W, Zhou Y, Lin J, Yan H, Huang H, Liu L, Qian J. Wang Y, et al. Emerg Microbes Infect. 2024 Dec;13(1):2368217. doi: 10.1080/22221751.2024.2368217. Epub 2024 Jul 5. Emerg Microbes Infect. 2024. PMID: 38865205 Free PMC article. - iGATE analysis improves the interpretability of single-cell immune landscape of influenza infection.
Hill BD, Zak AJ, Raja S, Bugada LF, Rizvi SM, Roslan SB, Nguyen HN, Chen J, Jiang H, Ono A, Goldstein DR, Wen F. Hill BD, et al. JCI Insight. 2024 May 30;9(12):e172140. doi: 10.1172/jci.insight.172140. JCI Insight. 2024. PMID: 38814732 Free PMC article.
References
- Fiore AE, et al. Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) Prevention and control of influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57:1–60. - PubMed
- Cox NJ, Neumann G, Donis RO, Kawaoka Y. Orthomyxoviruses: Influenza. In: Mahy BWJ, ter Meulen V, editors. Topley and Wilson's Microbiology and Microbial Infections. London, UK: Arnold; 2005. pp. 634–698.
- Palese P, Shaw ML. Orthomyxoviridae: The viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. 5th Ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 1647–1689.
- von Itzstein M. The war against influenza: Discovery and development of sialidase inhibitors. Nat Rev Drug Discov. 2007;6:967–974. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01AI058113/AI/NIAID NIH HHS/United States
- R01 AI046954/AI/NIAID NIH HHS/United States
- U19 AI083025/AI/NIAID NIH HHS/United States
- R01AI046954/AI/NIAID NIH HHS/United States
- U19AI083025/AI/NIAID NIH HHS/United States
- HHSN266200700010C/AI/NIAID NIH HHS/United States
- P01 AI058113/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous