Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders - PubMed (original) (raw)
. 2010 Jun 22;107(25):11459-64.
doi: 10.1073/pnas.1002443107. Epub 2010 Jun 7.
Gianpiero L Cavalleri, Libin Deng, Robert C Elston, Yang Gao, Jo Knight, Chaohua Li, Jiang Chuan Li, Yu Liang, Mark McCormack, Hugh E Montgomery, Hao Pan, Peter A Robbins, Kevin V Shianna, Siu Cheung Tam, Ngodrop Tsering, Krishna R Veeramah, Wei Wang, Puchung Wangdui, Michael E Weale, Yaomin Xu, Zhe Xu, Ling Yang, M Justin Zaman, Changqing Zeng, Li Zhang, Xianglong Zhang, Pingcuo Zhaxi, Yong Tang Zheng
Affiliations
- PMID: 20534544
- PMCID: PMC2895075
- DOI: 10.1073/pnas.1002443107
Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders
Cynthia M Beall et al. Proc Natl Acad Sci U S A. 2010.
Abstract
By impairing both function and survival, the severe reduction in oxygen availability associated with high-altitude environments is likely to act as an agent of natural selection. We used genomic and candidate gene approaches to search for evidence of such genetic selection. First, a genome-wide allelic differentiation scan (GWADS) comparing indigenous highlanders of the Tibetan Plateau (3,200-3,500 m) with closely related lowland Han revealed a genome-wide significant divergence across eight SNPs located near EPAS1. This gene encodes the transcription factor HIF2alpha, which stimulates production of red blood cells and thus increases the concentration of hemoglobin in blood. Second, in a separate cohort of Tibetans residing at 4,200 m, we identified 31 EPAS1 SNPs in high linkage disequilibrium that correlated significantly with hemoglobin concentration. The sex-adjusted hemoglobin concentration was, on average, 0.8 g/dL lower in the major allele homozygotes compared with the heterozygotes. These findings were replicated in a third cohort of Tibetans residing at 4,300 m. The alleles associating with lower hemoglobin concentrations were correlated with the signal from the GWADS study and were observed at greatly elevated frequencies in the Tibetan cohorts compared with the Han. High hemoglobin concentrations are a cardinal feature of chronic mountain sickness offering one plausible mechanism for selection. Alternatively, as EPAS1 is pleiotropic in its effects, selection may have operated on some other aspect of the phenotype. Whichever of these explanations is correct, the evidence for genetic selection at the EPAS1 locus from the GWADS study is supported by the replicated studies associating function with the allelic variants.
Conflict of interest statement
The authors are listed alphabetically and declare no conflict of interest.
Figures
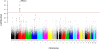
Fig. 1.
A genome-wide allelic differentiation scan that compares Tibetan residents at 3,200–3,500 m in Yunnan Province, China with HapMap Han samples. Eight SNPs near one another and EPAS1 have genome-wide significance. The horizontal axis is genomic position with colors indicating chromosomes. The vertical axis is the negative log of SNP-by-SNP P values generated from the Yunnan Tibetan vs. HapMap Han comparison. The red line indicates the threshold for genome-wide significance used (P = 5 × 10−7). Values are shown after correction for background population stratification using genomic control.
Fig. 2.
Sex-adjusted hemoglobin concentrations and allelic variation in EPAS1 SNPs in a Tibetan sample from Mag Xiang (4,200 m), Tibet Autonomous Region, China. Values were, on average, 0.8 g/dL lower for individuals who were homozygous for the major alleles compared with those who were heterozygous. (Top) The results of testing variants at 49 SNPs with a minor allele frequency ≥5% for genotypic association with sex-adjusted hemoglobin concentration. (Middle) The estimated hemoglobin concentration difference (mean ± 95% confidence interval) between the major allele homozygote and heterozygote genotypes at each SNP. Filled circles represent SNPs detected as having a significant association with hemoglobin concentration, with the false discovery rate controlled at <0.05 across the EPAS1 locus. Open diamonds represent SNPs without significant association. (Bottom) The pairwise linkage disequilibrium measured as _r_2 between SNPs.
Fig. 3.
Sex-adjusted hemoglobin concentrations and allelic variation in EPAS1 SNPs in a Tibetan sample from Zhaxizong Xiang (4,300 m), Tibet Autonomous Region, China. Values were, on average, 1.0 g/dL lower for individuals who were homozygous for the major alleles compared with those who were heterozygous (Top) The results of testing variants at 45 SNPs with a minor allele frequency ≥5% for genotypic association with sex-adjusted hemoglobin concentration. (Middle) The estimated hemoglobin concentration difference (mean ± 95% confidence interval) between the major allele homozygote and heterozygote genotypes at each SNP. Filled circles represent SNP detected as having a significant association with hemoglobin concentration with the false discovery rate controlled at <0.05 across the EPAS1 locus. Open diamonds represent SNP without significant association. (Bottom) The pairwise linkage disequilibrium measured as _r_2 between SNPs.
Fig. 4.
Differences in allelic frequency at SNPs within EPAS1 between the HapMap Han, Mag Xiang and Zhaxizong Xiang cohorts. The horizontal axis is SNP position according to build 36.1. The vertical axis is allelic frequency, with the allele selected for display as the one occurring most frequently in the Mag Xiang cohort. Squares denote data for HapMap Han; circles denote data for Mag Xiang Tibetans; triangles denote data for Zhaxizong Xiang Tibetans. Filled symbols denote those SNPs having significant associations with hemoglobin in both Mag Xiang and Zhaxizong Xiang cohorts; open symbols denote those SNPs without both such associations.
Comment in
- Will blood tell? Three recent articles demonstrate genetic selection in Tibetans.
Rupert J. Rupert J. High Alt Med Biol. 2010 Winter;11(4):307-8. doi: 10.1089/ham.2010.1052. High Alt Med Biol. 2010. PMID: 21190496 No abstract available.
Similar articles
- Down-Regulation of EPAS1 Transcription and Genetic Adaptation of Tibetans to High-Altitude Hypoxia.
Peng Y, Cui C, He Y, Ouzhuluobu, Zhang H, Yang D, Zhang Q, Bianbazhuoma, Yang L, He Y, Xiang K, Zhang X, Bhandari S, Shi P, Yangla, Dejiquzong, Baimakangzhuo, Duojizhuoma, Pan Y, Cirenyangji, Baimayangji, Gonggalanzi, Bai C, Bianba, Basang, Ciwangsangbu, Xu S, Chen H, Liu S, Wu T, Qi X, Su B. Peng Y, et al. Mol Biol Evol. 2017 Apr 1;34(4):818-830. doi: 10.1093/molbev/msw280. Mol Biol Evol. 2017. PMID: 28096303 Free PMC article. - EPAS1 and EGLN1 associations with high altitude sickness in Han and Tibetan Chinese at the Qinghai-Tibetan Plateau.
Buroker NE, Ning XH, Zhou ZN, Li K, Cen WJ, Wu XF, Zhu WZ, Scott CR, Chen SH. Buroker NE, et al. Blood Cells Mol Dis. 2012 Aug 15;49(2):67-73. doi: 10.1016/j.bcmd.2012.04.004. Epub 2012 May 15. Blood Cells Mol Dis. 2012. PMID: 22595196 - Gain-of-function EGLN1 prolyl hydroxylase (PHD2 D4E:C127S) in combination with EPAS1 (HIF-2α) polymorphism lowers hemoglobin concentration in Tibetan highlanders.
Tashi T, Scott Reading N, Wuren T, Zhang X, Moore LG, Hu H, Tang F, Shestakova A, Lorenzo F, Burjanivova T, Koul P, Guchhait P, Wittwer CT, Julian CG, Shah B, Huff CD, Gordeuk VR, Prchal JT, Ge R. Tashi T, et al. J Mol Med (Berl). 2017 Jun;95(6):665-670. doi: 10.1007/s00109-017-1519-3. Epub 2017 Feb 23. J Mol Med (Berl). 2017. PMID: 28233034 - Human adaptation to the hypoxia of high altitude: the Tibetan paradigm from the pregenomic to the postgenomic era.
Petousi N, Robbins PA. Petousi N, et al. J Appl Physiol (1985). 2014 Apr 1;116(7):875-84. doi: 10.1152/japplphysiol.00605.2013. Epub 2013 Nov 7. J Appl Physiol (1985). 2014. PMID: 24201705 Free PMC article. Review. - Adaptive genetic changes related to haemoglobin concentration in native high-altitude Tibetans.
Simonson TS, Huff CD, Witherspoon DJ, Prchal JT, Jorde LB. Simonson TS, et al. Exp Physiol. 2015 Nov;100(11):1263-8. doi: 10.1113/EP085035. Exp Physiol. 2015. PMID: 26454145 Review.
Cited by
- Archaic introgression contributed to shape the adaptive modulation of angiogenesis and cardiovascular traits in human high-altitude populations from the Himalayas.
Ferraretti G, Abondio P, Alberti M, Dezi A, Sherpa PT, Cocco P, Tiriticco M, Di Marcello M, Gnecchi-Ruscone GA, Natali L, Corcelli A, Marinelli G, Peluzzi D, Sarno S, Sazzini M. Ferraretti G, et al. Elife. 2024 Nov 8;12:RP89815. doi: 10.7554/eLife.89815. Elife. 2024. PMID: 39513938 Free PMC article. - A history of multiple Denisovan introgression events in modern humans.
Ongaro L, Huerta-Sanchez E. Ongaro L, et al. Nat Genet. 2024 Nov 5. doi: 10.1038/s41588-024-01960-y. Online ahead of print. Nat Genet. 2024. PMID: 39501127 Review. - Sex differences in genotype frequency and the risk of polycythemia associated with rs13419896 and rs2790859 among Tibetan highlanders living in Tsarang, Mustang, Nepal.
Arima H, Nishimura T, Koirala S, Nakano M, Ito H, Ichikawa T, Pandey K, Pandey BD, Yamamoto T. Arima H, et al. J Physiol Anthropol. 2024 Oct 15;43(1):25. doi: 10.1186/s40101-024-00372-5. J Physiol Anthropol. 2024. PMID: 39407294 Free PMC article. - EPAS1 Attenuates Atherosclerosis Initiation at Disturbed Flow Sites Through Endothelial Fatty Acid Uptake.
Pirri D, Tian S, Tardajos-Ayllon B, Irving SE, Donati F, Allen SP, Mammoto T, Vilahur G, Kabir L, Bennett J, Rasool Y, Pericleous C, Mazzei G, McAllan L, Scott WR, Koestler T, Zingg U, Birdsey GM, Miller CL, Schenkel T, Chambers EV, Dunning MJ, Serbanovic-Canic J, Botrè F, Mammoto A, Xu S, Osto E, Han W, Fragiadaki M, Evans PC. Pirri D, et al. Circ Res. 2024 Sep 27;135(8):822-837. doi: 10.1161/CIRCRESAHA.123.324054. Epub 2024 Sep 5. Circ Res. 2024. PMID: 39234692 Free PMC article. - Evolution of Key Oxygen-Sensing Genes Is Associated with Hypoxia Tolerance in Fishes.
Babin CH, Leiva FP, Verberk WCEP, Rees BB. Babin CH, et al. Genome Biol Evol. 2024 Sep 3;16(9):evae183. doi: 10.1093/gbe/evae183. Genome Biol Evol. 2024. PMID: 39165136 Free PMC article.
References
- Aldenderfer M. Moving up in the world: Archaeologists seek to understand how and when people came to occupy the Andean and Tibetan plateaus. Am Sci. 2003;91:542–549.
- Wu T, Miao C. High altitude heart disease in children in Tibet. High Alt Med Biol. 2002;3:323–325. - PubMed
- León-Velarde F, et al. Consensus statement on chronic and subacute high altitude diseases. High Alt Med Biol. 2005;6:147–157. - PubMed
- Wu T. Life on the high Tibetan plateau. High Alt Med Biol. 2004;5:1–2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical