Exploiting mosquito sugar feeding to detect mosquito-borne pathogens - PubMed (original) (raw)
Exploiting mosquito sugar feeding to detect mosquito-borne pathogens
Sonja Hall-Mendelin et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Arthropod-borne viruses (arboviruses) represent a global public health problem, with dengue viruses causing millions of infections annually, while emerging arboviruses, such as West Nile, Japanese encephalitis, and chikungunya viruses have dramatically expanded their geographical ranges. Surveillance of arboviruses provides vital data regarding their prevalence and distribution that may be utilized for biosecurity measures and the implementation of disease control strategies. However, current surveillance methods that involve detection of virus in mosquito populations or sero-conversion in vertebrate hosts are laborious, expensive, and logistically problematic. We report a unique arbovirus surveillance system to detect arboviruses that exploits the process whereby mosquitoes expectorate virus in their saliva during sugar feeding. In this system, infected mosquitoes captured by CO(2)-baited updraft box traps are allowed to feed on honey-soaked nucleic acid preservation cards within the trap. The cards are then analyzed for expectorated virus using real-time reverse transcription-PCR. In field trials, this system detected the presence of Ross River and Barmah Forest viruses in multiple traps deployed at two locations in Australia. Viral RNA was preserved for at least seven days on the cards, allowing for long-term placement of traps and continuous collection of data documenting virus presence in mosquito populations. Furthermore no mosquito handling or processing was required and cards were conveniently shipped to the laboratory overnight. The simplicity and efficacy of this approach has the potential to transform current approaches to vector-borne disease surveillance by streamlining the monitoring of pathogens in vector populations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Updraft box trap use to collect and house mosquitoes. (A) Trap components and direction of airflow depicted by solid arrows. Mosquitoes are sucked into the trap at the bottom, then become trapped within the plastic box (B) where they can feed on honey-soaked FTA® cards (C). The front of the trap has been removed for photographic purposes.
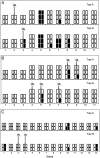
Fig. 2.
Temporal depiction of the detection of arboviruses in honey-soaked FTA® cards and mosquito pools collected from duplicate CO2-baited updraft box traps in the field: (A) Ross River virus and (B) Barmah Forest virus from the Leschenault Peninsula near Bunbury in Western Australia, and (C) Ross River virus from Cairns, far north Queensland. Each square represents a single FTA® card that was processed in each week, and black squares indicate a card from which viral RNA was detected. Detections of viral RNA in mosquitoes removed from the updraft box traps at the same time as the FTA® cards are represented by a mosquito.
Similar articles
- Converting Mosquito Surveillance to Arbovirus Surveillance with Honey-Baited Nucleic Acid Preservation Cards.
Flies EJ, Toi C, Weinstein P, Doggett SL, Williams CR. Flies EJ, et al. Vector Borne Zoonotic Dis. 2015 Jul;15(7):397-403. doi: 10.1089/vbz.2014.1759. Vector Borne Zoonotic Dis. 2015. PMID: 26186511 - Applications of a sugar-based surveillance system to track arboviruses in wild mosquito populations.
van den Hurk AF, Hall-Mendelin S, Townsend M, Kurucz N, Edwards J, Ehlers G, Rodwell C, Moore FA, McMahon JL, Northill JA, Simmons RJ, Cortis G, Melville L, Whelan PI, Ritchie SA. van den Hurk AF, et al. Vector Borne Zoonotic Dis. 2014 Jan;14(1):66-73. doi: 10.1089/vbz.2013.1373. Epub 2013 Dec 20. Vector Borne Zoonotic Dis. 2014. PMID: 24359415 - Evaluation of honey-baited FTA cards in combination with different mosquito traps in an area of low arbovirus prevalence.
Wipf NC, Guidi V, Tonolla M, Ruinelli M, Müller P, Engler O. Wipf NC, et al. Parasit Vectors. 2019 Nov 21;12(1):554. doi: 10.1186/s13071-019-3798-8. Parasit Vectors. 2019. PMID: 31753035 Free PMC article. - Evolution of mosquito-based arbovirus surveillance systems in Australia.
van den Hurk AF, Hall-Mendelin S, Johansen CA, Warrilow D, Ritchie SA. van den Hurk AF, et al. J Biomed Biotechnol. 2012;2012:325659. doi: 10.1155/2012/325659. Epub 2012 Mar 11. J Biomed Biotechnol. 2012. PMID: 22505808 Free PMC article. Review. - Searching for the proverbial needle in a haystack: advances in mosquito-borne arbovirus surveillance.
Ramírez AL, van den Hurk AF, Meyer DB, Ritchie SA. Ramírez AL, et al. Parasit Vectors. 2018 May 29;11(1):320. doi: 10.1186/s13071-018-2901-x. Parasit Vectors. 2018. PMID: 29843778 Free PMC article. Review.
Cited by
- Excretion of dengue virus RNA by Aedes aegypti allows non-destructive monitoring of viral dissemination in individual mosquitoes.
Fontaine A, Jiolle D, Moltini-Conclois I, Lequime S, Lambrechts L. Fontaine A, et al. Sci Rep. 2016 Apr 27;6:24885. doi: 10.1038/srep24885. Sci Rep. 2016. PMID: 27117953 Free PMC article. - The Transcriptional Response of Aedes aegypti with Variable Extrinsic Incubation Periods for Dengue Virus.
Koh C, Allen SL, Herbert RI, McGraw EA, Chenoweth SF. Koh C, et al. Genome Biol Evol. 2018 Dec 1;10(12):3141-3151. doi: 10.1093/gbe/evy230. Genome Biol Evol. 2018. PMID: 30335126 Free PMC article. - Aptamer-gold nanoparticle conjugates for the colorimetric detection of arboviruses and vector mosquito species.
Bosak A, Saraf N, Willenberg A, Kwan MWC, Alto BW, Jackson GW, Batchelor RH, Nguyen-Huu TD, Sankarapani V, Parks GD, Seal S, Willenberg BJ. Bosak A, et al. RSC Adv. 2019 Jul 31;9(41):23752-23763. doi: 10.1039/c9ra02089f. eCollection 2019 Jul 29. RSC Adv. 2019. PMID: 35530619 Free PMC article. - Putative novel lineage of West Nile virus in Uranotaenia unguiculata mosquito, Hungary.
Kemenesi G, Dallos B, Oldal M, Kutas A, Földes F, Németh V, Reiter P, Bakonyi T, Bányai K, Jakab F. Kemenesi G, et al. Virusdisease. 2014 Dec;25(4):500-3. doi: 10.1007/s13337-014-0234-8. Epub 2014 Oct 24. Virusdisease. 2014. PMID: 25674630 Free PMC article. - Use of scented sugar bait stations to track mosquito-borne arbovirus transmission in California.
Lothrop HD, Wheeler SS, Fang Y, Reisen WK. Lothrop HD, et al. J Med Entomol. 2012 Nov;49(6):1466-72. doi: 10.1603/me12117. J Med Entomol. 2012. PMID: 23270177 Free PMC article.
References
- Gould EA, Solomon T. Pathogenic flaviviruses. Lancet. 2008;371:500–509. - PubMed
- Staples JE, Breiman RF, Powers AM. Chikungunya fever: An epidemiological review of a re-emerging infectious disease. Clin Infect Dis. 2009;49:942–948. - PubMed
- Eisen L, Beaty BJ, Morrison AC, Scott TW. Proactive vector control strategies and improved monitoring and evaluation practices for dengue prevention. J Med Entomol. 2009;46:1245–1255. - PubMed
- Hanna JN, et al. Japanese encephalitis in north Queensland, Australia, 1998. Med J Aust. 1999;170:533–536. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources