Afferent-specific AMPA receptor subunit composition and regulation of synaptic plasticity in midbrain dopamine neurons by abused drugs - PubMed (original) (raw)
Afferent-specific AMPA receptor subunit composition and regulation of synaptic plasticity in midbrain dopamine neurons by abused drugs
Cameron H Good et al. J Neurosci. 2010.
Abstract
Ventral tegmental area (VTA) dopamine (DA) neurons play a pivotal role in processing reward-related information and are involved in drug addiction and mental illness in humans. Information is conveyed to the VTA in large part by glutamatergic afferents that arise in various brain nuclei, including the pedunculopontine nucleus (PPN). Using a unique rat brain slice preparation, we found that PPN stimulation activates afferents targeting GluR2-containing AMPA receptors (AMPAR) on VTA DA neurons, and these afferents did not exhibit long-term depression (LTD). In contrast, activation of glutamate afferents onto the same DA neurons via stimulation within the VTA evoked EPSCs mediated by GluR2-lacking AMPARs that demonstrated LTD or EPSCs mediated by GluR2-containing AMPA receptors that did not express LTD. Twenty-four hours after single cocaine injections to rats, GluR2-lacking AMPARs were increased at both PPN and local VTA projections, and this permitted LTD expression in both pathways. Single injections with the main psychoactive ingredient of marijuana, Delta(9)-tetrahydrocannabinol (Delta(9)-THC), increased GluR2-lacking AMPA receptors and permitted LTD in only the PPN pathway, and these effects were prevented by in vivo pretreatment with the cannabinoid CB1 receptor antagonist AM251. These results demonstrate that cocaine more globally increases GluR2-lacking AMPA receptors at all glutamate synapses on VTA dopamine neurons, whereas Delta(9)-THC selectively increased GluR2-lacking AMPA receptors at subcortical PPN synapses. This suggests that different abused drugs may exert influence over distinct sets of glutamatergic afferents to VTA DA neurons which may be associated with different reinforcing or addictive properties of these drugs.
Figures
Figure 1.
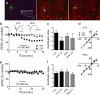
JTx inhibition of EPSCs evoked via intra-VTA, but not PPN stimulation, indicates the absence or presence of the GluR2 AMPAR subunit, respectively, at these synapses on VTA DA neurons. a, Photomicrograph of a biocytin-filled (left) VTA neuron that expressed the DAT (center, arrow), and a superimposition of these images (right). Also shown are membrane currents elicited by hyperpolarizing voltage steps, indicating robust _I_h (left, inset; scale bars, 200 ms and 200 pA). Scale bar, 20 μm. b, Mean time course of JTx (horizontal bar) effects on EPSCs evoked via intra-VTA or PPN stimulation in the same VTA DA neurons (n = 14). Inset, Representative EPSCs during control and JTx application (n = 3–5 EPSCs per average; scale bars, 10 ms, 100 pA). Data shown in b represent only neurons in which intra-VTA-evoked EPSCs were inhibited by JTx (16/25, 64%). e, Absence of JTx effect on intra-VTA or PPN-evoked EPSCs in a subpopulation of VTA DA neurons (9/25, 36%). c, RI of EPSCs evoked via intra-VTA or PPN stimulation, before and during JTx application. There was a significant decrease in RI of the intra-VTA (paired t test, p < 0.01), but not the PPN-evoked, EPSCs in a subset of cells, whereas there was no change in the RI measured at either pathway in the remaining neurons (f, ANOVA, ns). d, I–V curves measured before and after JTx application from the same cells as in b and c.
Figure 2.
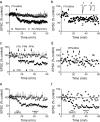
Selective LTD expression at intra-VTA-activated glutamate synapses. a, Mean time course showing the effects of 66 Hz stimulation of the anterior VTA on AMPAR EPSCs in all VTA DA neurons. Intra-VTA-evoked LTD was observed in 11 of 25 cells (44%, Responders). b, Time course of LTD in a representative VTA DA neuron. Inset, Mean EPSCs before and after 66 Hz stimulation of the VTA (scale bars, 10 ms, 200 pA). c, Absence of 66 Hz LTD (stimulation applied to the respective pathways at arrows) in the PPN-activated pathway, in the same VTA DA neurons demonstrating intra-VTA-initiated LTD. The 66 Hz stimulation was applied through the PPN electrode only when LTD was observed first in the intra-VTA pathway. LTD was never observed in the PPN pathway following 66 Hz stimulation of the VTA, nor following multiple bouts of 66 Hz stimulation to the PPN (arrows; d). d, Representative neuron demonstrating no effect of repeated 66 Hz stimulation of the PPN on either PPN- or intra-VTA-evoked EPSCs. e, Mean time course of the effects of brief DHPG application (horizontal bar) on intra-VTA and PPN-evoked EPSCs in the same VTA DA neurons. JTx (500 n
m
), applied after DHPG washout, had no effect on either PPN or intra-VTA-evoked EPSCs, confirming the presence of GluR2-containing AMPARs at intra-VTA-activated synapses after LTD (compare with Fig. 1_b_). f, Representative example of a cell in which DHPG caused robust LTD of the intra-VTA pathway, but had no effect on the PPN stimulated pathway, and the absence of a post-LTD JTx effect.
Figure 3.
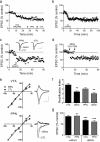
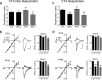
A single cocaine injection changes AMPAR subunit composition, leading to reliable LTD at both intra-VTA- and PPN-activated synapses in the same VTA DA neurons. a, Mean time course of the effect of the JTx analog NASPM (horizontal bar) on EPSCs evoked via both pathways. PPN and the intra-VTA-activated glutamate synapses were inhibited by NASPM in all neurons, indicating a loss of GluR2-AMPARs following cocaine exposure. b, Mean time course of DHPG-induced LTD of EPSCs evoked via both pathways (n = 6). LTD was observed at intra-VTA- and PPN-activated synapses in 100% of the cells 24 h after the cocaine injection. c, Representative time course and EPSCs from a single DA neuron showing DHPG-LTD in both pathways (scale bars, 5 ms, 100 pA for VTA-evoked, and 5 ms, 25 pA for PPN-evoked EPSCs). d, Mean time course showing the effect of 66 Hz stimulation applied to the intra-VTA pathway, followed by PPN stimulation 10 min later (n = 8). e, Inward rectification of EPSCs in both pathways 24 h following a single cocaine exposure (CTL), and subsequent loss of rectification following 66 Hz stimulation in both pathways. EPSCs were normalized to those recorded at −80 mV. Example wave forms are shown at +40 mV and −80 mV before and after 66 Hz stimulation in each pathway (scale bars, 5 ms, 200 pA, VTA pathway; 5 ms, 50 pA, PPN pathway). f, Mean RI before and after 66 Hz stimulation of the intra-VTA- or PPN-activated inputs to the same VTA DA neurons. g, Mean EPSC amplitude changes in both pathways at +40 mV and −80 mV after 66 Hz stimulation, normalized to pre-66 Hz EPSC amplitudes (−80 mV, ANOVA, **p < 0.01, ***p < 0.0001).
Figure 4.
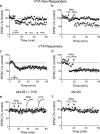
Afferent-specific LTD at PPN-glutamate synapses following a single exposure to Δ9-THC. a, Mean time course showing that DHPG initiates LTD of PPN-evoked EPSCs (n = 16/16), but not of intra-VTA-evoked EPSCs, in the same subpopulation of VTA DA neurons 24 h after a single injection of Δ9-THC (10 mg/kg, i.p.). b, Mean time course showing 66 Hz LTD of PPN-evoked EPSCs, but not intra-VTA-evoked EPSCs in a subpopulation of VTA DA neurons. c, DHPG-induced LTD of both pathways following a single injection of Δ9-THC in a subpopulation of VTA DA neurons. d, Mean time course of 66 Hz LTD in both pathways in a subpopulation of neurons. e, The CB1R antagonist AM251, injected 30 min before Δ9-THC blocks 66 Hz LTD. f, Mean time course of the effect of 66 Hz stimulation of both pathways in VTA DA neurons from animals receiving a single injection of AM251. In this experiment 6/14 (43%) intra-VTA-evoked EPSCs demonstrated 66 Hz LTD, whereas 0/9 cells demonstrated LTD following 66 Hz stimulation of the PPN pathway.
Figure 5.
A single injection of Δ9-THC leads to a pathway-specific change in the subunit composition of synaptic AMPARs. a, The RI of intra-VTA-activated EPSCS is not altered by 66 Hz stimulation following Δ9-THC administration, in a subset of VTA DA neurons (VTA nonresponders, 9/20 cells). However, in the same cells, the RI of all PPN-activated EPSCs was significantly decreased following 66 Hz-induced LTD. b, EPSC I–V curves from the PPN and intra-VTA glutamate pathways before and after 66 Hz stimulation (scale bars, 5 ms, 200 pA for VTA examples; 5 ms, 100 pA for PPN examples). c, In another group of cells (VTA Responders, 11/20), both the VTA and PPN undergo significant changes in the RI following 66 Hz stimulation. d, EPSC I–V curves in both the PPN and VTA pathways before and after 66 Hz stimulation. Note that both pathways exhibit linear I–V curves after 66 Hz-induced LTD. Scale bars are 5 ms and 200 pA for the VTA examples and 5 ms and 100 pA for the PPN examples; **p < 0.01, ***p < 0.001, paired t test.
Figure 6.
Compilation of the effects of all manipulations on EPSC amplitudes for all neurons included in this study. The numbers in parentheses represent the total number of VTA DA neurons in which observations were made during each manipulation. Gray circles indicate EPSCs evoked by stimulation of the PPN, whereas, black circles represent EPSCs evoked via stimulation of the rostral VTA (intra-VTA). “Control” indicates either no injection 24 h before brain slice preparation, or those responses from animals receiving a single saline injection. Note that PPN-evoked EPSCs were insensitive to JTx and did not demonstrate 66 Hz-, or DHPG-induced LTD unless measured in VTA DA neurons obtained from cocaine- or Δ9-THC-treated animals. In addition, Δ9-THC exposure did not appear to increase the likelihood of intra-VTA-evoked LTD expression above that observed under control conditions.
Similar articles
- Cocaine Selectively Reorganizes Excitatory Inputs to Substantia Nigra Pars Compacta Dopamine Neurons.
Beaudoin GMJ 3rd, Gomez JA, Perkins J, Bland JL, Petko AK, Paladini CA. Beaudoin GMJ 3rd, et al. J Neurosci. 2018 Jan 31;38(5):1151-1159. doi: 10.1523/JNEUROSCI.1975-17.2017. Epub 2017 Dec 20. J Neurosci. 2018. PMID: 29263240 Free PMC article. - Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area.
Mameli M, Balland B, Luján R, Lüscher C. Mameli M, et al. Science. 2007 Jul 27;317(5837):530-3. doi: 10.1126/science.1142365. Science. 2007. PMID: 17656725 - Synaptic and intrinsic plasticity in the ventral tegmental area after chronic cocaine.
Francis TC, Gantz SC, Moussawi K, Bonci A. Francis TC, et al. Curr Opin Neurobiol. 2019 Feb;54:66-72. doi: 10.1016/j.conb.2018.08.013. Epub 2018 Sep 17. Curr Opin Neurobiol. 2019. PMID: 30237117 Free PMC article. Review. - Cocaine-evoked synaptic plasticity of excitatory transmission in the ventral tegmental area.
Lüscher C. Lüscher C. Cold Spring Harb Perspect Med. 2013 May 1;3(5):a012013. doi: 10.1101/cshperspect.a012013. Cold Spring Harb Perspect Med. 2013. PMID: 23637310 Free PMC article. Review.
Cited by
- Repeated exposure to methamphetamine, cocaine or morphine induces augmentation of dopamine release in rat mesocorticolimbic slice co-cultures.
Nakagawa T, Suzuki Y, Nagayasu K, Kitaichi M, Shirakawa H, Kaneko S. Nakagawa T, et al. PLoS One. 2011;6(9):e24865. doi: 10.1371/journal.pone.0024865. Epub 2011 Sep 30. PLoS One. 2011. PMID: 21980362 Free PMC article. - Glutamate inputs from the laterodorsal tegmental nucleus to the ventral tegmental area are essential for the induction of cocaine sensitization in male mice.
Puranik A, Buie N, Arizanovska D, Vezina P, Steidl S. Puranik A, et al. Psychopharmacology (Berl). 2022 Oct;239(10):3263-3276. doi: 10.1007/s00213-022-06209-2. Epub 2022 Aug 25. Psychopharmacology (Berl). 2022. PMID: 36006414 - Unraveling glutamate-opioid receptor interactions using high-resolution electron microscopy: implications for addiction-related processes.
Scavone JL, Asan E, Van Bockstaele EJ. Scavone JL, et al. Exp Neurol. 2011 Jun;229(2):207-13. doi: 10.1016/j.expneurol.2011.03.016. Epub 2011 Apr 1. Exp Neurol. 2011. PMID: 21459090 Free PMC article. - Rehabilitating the addicted brain with transcranial magnetic stimulation.
Diana M, Raij T, Melis M, Nummenmaa A, Leggio L, Bonci A. Diana M, et al. Nat Rev Neurosci. 2017 Nov;18(11):685-693. doi: 10.1038/nrn.2017.113. Epub 2017 Sep 29. Nat Rev Neurosci. 2017. PMID: 28951609 Review. - Inhibition of Protein kinase Mzeta (PKMζ) in the mesolimbic system alters cocaine sensitization in rats.
Vélez-Hernández ME, Vázquez-Torres R, Velasquez-Martinez MC, Jiménez L, Báez F, Sacktor TC, Jiménez-Rivera CA. Vélez-Hernández ME, et al. J Drug Alcohol Res. 2013 Jul 1;2:235669. doi: 10.4303/jdar/235669. J Drug Alcohol Res. 2013. PMID: 24729912 Free PMC article.
References
- Bellone C, Lüscher C. mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. Eur J Neurosci. 2005;21:1280–1288. - PubMed
- Bellone C, Lüscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources