Vesicular monoamine and glutamate transporters select distinct synaptic vesicle recycling pathways - PubMed (original) (raw)
Vesicular monoamine and glutamate transporters select distinct synaptic vesicle recycling pathways
Bibiana Onoa et al. J Neurosci. 2010.
Abstract
Previous work has characterized the properties of neurotransmitter release at excitatory and inhibitory synapses, but we know remarkably little about the properties of monoamine release, because these neuromodulators do not generally produce a fast ionotropic response. Since dopamine and serotonin neurons can also release glutamate in vitro and in vivo, we have used the vesicular monoamine transporter VMAT2 and the vesicular glutamate transporter VGLUT1 to compare the localization and recycling of synaptic vesicles that store, respectively, monoamines and glutamate. First, VMAT2 segregates partially from VGLUT1 in the boutons of midbrain dopamine neurons, indicating the potential for distinct release sites. Second, endocytosis after stimulation is slower for VMAT2 than VGLUT1. During the stimulus, however, the endocytosis of VMAT2 (but not VGLUT1) accelerates dramatically in midbrain dopamine but not hippocampal neurons, indicating a novel, cell-specific mechanism to sustain high rates of release. On the other hand, we find that in both midbrain dopamine and hippocampal neurons, a substantially smaller proportion of VMAT2 than VGLUT1 is available for evoked release, and VMAT2 shows considerably more dispersion along the axon after exocytosis than VGLUT1. Even when expressed in the same neuron, the two vesicular transporters thus target to distinct populations of synaptic vesicles, presumably due to their selection of distinct recycling pathways.
Figures
Figure 1.
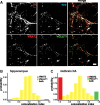
VMAT2 and VGLUT1 localize to the same terminals in hippocampal neurons but differently in midbrain dopamine neurons. A, Representative fluorescence images of midbrain neurons transfected with a bicistronic plasmid encoding both GFP-VGLUT1 and HA-VMAT2. GFP-VGLUT1 was stained for GFP using a secondary antibody conjugated to Cy2 (shown in green), and HA-VMAT2 for HA with secondary conjugated to Cy3 (red). To identify presynaptic sites, endogenous synaptophysin (syp) was labeled using an Alexa 405 secondary antibody (blue). To identify dopamine neurons, the cultures were stained for TH using a secondary antibody conjugated to Cy5 (brown). Arrowheads indicate colocalization of VGLUT1 and VMAT2 at syp+, TH+ sites, and the arrow indicates VMAT2 without VGLUT1. Scale bar, 5 μm. B, The extent of VGLUT1 and VMAT2 colocalization was quantified at presynaptic terminals of hippocampal neurons triple stained for endogenous synaptophysin and introduced VGLUT1 (green) and VMAT2 (red). The colocalization index and distribution of intensity ratios indicate that ∼64% of hippocampal terminals show >90% colocalization, and <2% do not colocalize (red or green bars). **_C_**, In midbrain dopamine neurons, the distribution of colocalization index indicates that 37% of terminals show >90% colocalization and ∼27% do not colocalize (382–434 boutons, 3–4 coverslips, and 1–2 transfections per condition).
Figure 2.
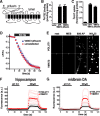
Development of a pHluorin-based reporter for VMAT2. A, The superecliptic pHluorin was inserted into the first lumenal loop of VMAT2 using a combination of different linkers (supplemental Fig. 1_A_, available at
as supplemental material). The resulting cDNAs were transfected into COS cells and the extracts tested for expression by Western analysis, for binding to the noncompetitive inhibitor TBZ, and for the uptake of 3H-serotonin. One of the two constructs that met these criteria (VMAT2-pHluorin) was then compared directly to a fusion of GFP to the N terminus of VMAT2 (GFP-VMAT2) in terms of TBZ binding (B) and serotonin uptake (C). D, Transfected into primary hippocampal culture, VMAT2-pHluorin does not affect the destaining of preloaded FM4-64 by 10 Hz stimulation for 60 s. E, Hippocampal neurons transfected with either VGLUT1- or VMAT2-pHluorin were imaged at baseline (rest), upon acid quenching in MES buffer, pH 5.5 for 20 s, at the end of stimulation with 600 action potentials (600 AP), and upon total alkalinization for 20 s in 50 m
m
NH4Cl, pH 7.4. F, Bouton fluorescence in hippocampal neurons transfected with VMAT2 (n = 12 coverslips from 3 cultures containing a total of 418 boutons) or VGLUT1 (n = 8 coverslips from 3 cultures containing 194 boutons) shows very low surface expression of the two transporters, but substantially higher total expression of VMAT2 than VGLUT1 after alkalinization with NH4Cl. G, The surface and total expression of VGLUT1 (n = 6 coverslips from 4 cultures containing a total of 253 boutons) and VMAT2 (n = 8 coverslips for 3 cultures containing 274 boutons) exhibited similar behavior in transfected midbrain dopamine neurons. Light traces in F and G indicate the coverslip means, and dark traces the means of the light traces.
Figure 3.
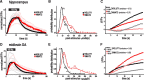
The kinetics of endocytosis differ between VMAT2 and VGLUT1 in both hippocampal and midbrain dopamine neurons. A, Time course of VGLUT1-pHluorin and VMAT2-pHluorin fluorescence changes produced by 10 Hz stimulation for 60 s in hippocampal neurons, normalized to the maximum fluorescence. Fitting the fluorescence decay to a single exponential, the mean poststimulus τendo in terminals expressing VMAT2 is larger than in those expressing VGLUT1 (p = 0.015; n = 5–6 coverslips from 3 cultures containing a total of 218–353 boutons). B, A histogram of poststimulus τendo shows a bimodal distribution for both transfected reporters that is well fitted by the sum of two Gaussians (R adjusted 2 > 0.92 for both fittings), and indicates that the poststimulus endocytosis of VGLUT1 is faster and more tightly distributed than that of VMAT2 in hippocampal neurons. C, The kinetics of exocytosis and endocytosis during stimulation were determined in the presence and absence of bafilomycin A1 (baf) for both VGLUT1 and VMAT2 reporters. Although VMAT2 is more highly expressed, the exocytosis of VGLUT1 is faster (p = 0.015; n = 4–6 coverslips from 2 cultures containing a total of 81–99 boutons). Despite a lag of 15–20 s, the endocytosis of VGLUT1 is also faster than VMAT2 (p = 0.003). D, The normalized response of VGLUT1 and VMAT2 to stimulation of transfected midbrain dopamine neurons resembles that observed in hippocampal neurons (A), with faster endocytosis of VGLUT1 (p = 0.06 the null hypothesis was rejected; n = 7 coverslips from 3–4 cultures containing a total of 207–269 boutons). E, In midbrain dopamine neurons, the distribution of poststimulus τendo shows that the endocytosis of VGLUT1 is faster than that of VMAT2. As in hippocampal neurons, τendo exhibits a bimodal distribution (R adjusted 2 > 0.94 for both fittings). F, During stimulation, VMAT2 in midbrain dopamine neurons (n = 3 coverslips containing 152 boutons from 2 cultures) exhibits more rapid endocytosis than VGLUT1 (n = 5 coverslips containing 134 boutons from 2 cultures) (p = 0.05), in contrast to the results in hippocampal neurons. Further, the initial lag in endocytosis observed in hippocampal neurons does not occur in midbrain neurons. Light traces in A and D indicate coverslip means, and dark traces the mean of light traces. In C and F, each trace indicates the mean of coverslip means, with coverslip means omitted for clarity.
Figure 4.
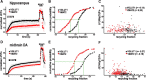
A smaller proportion of VMAT2 undergoes stimulated exocytosis than VGLUT1. A, Time course of fluorescence changes for hippocampal neurons transfected with VGLUT1 (n = 4 coverslips containing a total of 92 boutons from 2 cultures) or VMAT2-pHluorin cDNAs (n = 6 coverslips containing 125 boutons from 2 cultures) and stimulated at 10 Hz for 60 s in the presence of bafilomycin, normalized to total fluorescence in NH4Cl. A substantially smaller fraction of VMAT2 undergoes exocytosis in response to stimulation than VGLUT1 (p = 0.005). B, Cumulative probability distribution of the recycling fraction (ratio of maximal fluorescence at the end of stimulation to total fluorescence in NH4Cl). A cumulative probability of 50% corresponds to a recycling fraction of 0.46 in the case of VGLUT1 and 0.30 in the case of VMAT2 (green dotted lines). C, The recycling fraction determined does not correlate with the expression of reporter (revealed in NH4Cl). D, The analysis of midbrain dopamine neurons transfected with VGLUT1 (n = 4 coverslips containing 134 boutons from 2 cultures) or VMAT2 (n = 5 coverslips containing 226 boutons from 2 cultures) also shows a smaller recycling pool size for VMAT2 (p = 0.008), similar to hippocampal neurons. E, A cumulative probability of 50% corresponds to a recycling fraction of 0.33 for VGLUT1 and 0.22 for VMAT2. F, The recycling fraction does not correlate with reporter expression in midbrain dopamine neurons. In A and D, light traces indicate coverslip means and dark traces the means of the light traces.
Figure 5.
VMAT2 exhibits more stimulus-induced dispersion than VGLUT1. A, Time course of fluorescence changes for GFP-VGLUT1 (n = 5 coverslips containing 141 boutons from 2 cultures) and GFP-VMAT2 (n = 5 coverslips containing 138 boutons from 2 cultures) at the boutons of transfected hippocampal neurons stimulated at 10 Hz for 60 s. Light traces indicate coverslip means, and dark traces the means of those light traces. At boutons, the fluorescence of GFP-VMAT2 shows a larger decrement with stimulation than that of VGLUT1 (p = 0.02), and appears to recover more slowly, indicating greater dispersion and less reclustering. B, A histogram of the extent of fluorescence decrease at boutons (dispersion amplitude) shows that VMAT2 indeed shows greater dispersion than VGLUT1. C, A histogram of the extent of fluorescence recovery after stimulation shows that a larger percentage of VMAT2 boutons do not show any evidence of reclustering over this time interval. Inset shows sample traces (in blue) from selected boutons. D, The kinetics of reclustering was calculated by fitting to a single exponential function the traces that show fluorescence recovery after stimulation. Excluding boutons where the fluorescence does not recover, an average time course was plotted for those where recovery occurs (46% of boutons for VGLUT1 and 25% for VMAT2). Although the extent of reclustering in these traces is now very similar, the kinetics are not (p = 0.01).
Similar articles
- Normal biogenesis and cycling of empty synaptic vesicles in dopamine neurons of vesicular monoamine transporter 2 knockout mice.
Croft BG, Fortin GD, Corera AT, Edwards RH, Beaudet A, Trudeau LE, Fon EA. Croft BG, et al. Mol Biol Cell. 2005 Jan;16(1):306-15. doi: 10.1091/mbc.e04-07-0559. Epub 2004 Oct 20. Mol Biol Cell. 2005. PMID: 15496457 Free PMC article. - Regulation of dopamine quantal size in midbrain and hippocampal neurons.
Pothos EN. Pothos EN. Behav Brain Res. 2002 Mar 10;130(1-2):203-7. doi: 10.1016/s0166-4328(01)00419-3. Behav Brain Res. 2002. PMID: 11864736 - Protein interactions of the vesicular glutamate transporter VGLUT1.
Santos MS, Foss SM, Park CK, Voglmaier SM. Santos MS, et al. PLoS One. 2014 Oct 15;9(10):e109824. doi: 10.1371/journal.pone.0109824. eCollection 2014. PLoS One. 2014. PMID: 25334008 Free PMC article. - Protective actions of the vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons.
Guillot TS, Miller GW. Guillot TS, et al. Mol Neurobiol. 2009 Apr;39(2):149-70. doi: 10.1007/s12035-009-8059-y. Epub 2009 Mar 4. Mol Neurobiol. 2009. PMID: 19259829 Review. - Presynaptic regulation of dopamine release: Role of the DAT and VMAT2 transporters.
Mulvihill KG. Mulvihill KG. Neurochem Int. 2019 Jan;122:94-105. doi: 10.1016/j.neuint.2018.11.004. Epub 2018 Nov 19. Neurochem Int. 2019. PMID: 30465801 Review.
Cited by
- Corelease of dopamine and GABA by a retinal dopaminergic neuron.
Hirasawa H, Betensky RA, Raviola E. Hirasawa H, et al. J Neurosci. 2012 Sep 19;32(38):13281-91. doi: 10.1523/JNEUROSCI.2213-12.2012. J Neurosci. 2012. PMID: 22993444 Free PMC article. - Target-Specific Glycinergic Transmission from VGluT3-Expressing Amacrine Cells Shapes Suppressive Contrast Responses in the Retina.
Tien NW, Kim T, Kerschensteiner D. Tien NW, et al. Cell Rep. 2016 May 17;15(7):1369-1375. doi: 10.1016/j.celrep.2016.04.025. Epub 2016 May 5. Cell Rep. 2016. PMID: 27160915 Free PMC article. - Peripheral and central employment of acid-sensing ion channels during early bilaterian evolution.
Martí-Solans J, Børve A, Bump P, Hejnol A, Lynagh T. Martí-Solans J, et al. Elife. 2023 Feb 23;12:e81613. doi: 10.7554/eLife.81613. Elife. 2023. PMID: 36821351 Free PMC article. - The Synaptic Vesicle Cycle Revisited: New Insights into the Modes and Mechanisms.
Chanaday NL, Cousin MA, Milosevic I, Watanabe S, Morgan JR. Chanaday NL, et al. J Neurosci. 2019 Oct 16;39(42):8209-8216. doi: 10.1523/JNEUROSCI.1158-19.2019. J Neurosci. 2019. PMID: 31619489 Free PMC article. Review. - Fluorescent false neurotransmitter reveals functionally silent dopamine vesicle clusters in the striatum.
Pereira DB, Schmitz Y, Mészáros J, Merchant P, Hu G, Li S, Henke A, Lizardi-Ortiz JE, Karpowicz RJ Jr, Morgenstern TJ, Sonders MS, Kanter E, Rodriguez PC, Mosharov EV, Sames D, Sulzer D. Pereira DB, et al. Nat Neurosci. 2016 Apr;19(4):578-86. doi: 10.1038/nn.4252. Epub 2016 Feb 22. Nat Neurosci. 2016. PMID: 26900925 Free PMC article.
References
- Abeliovich A, Schmitz Y, Fariñas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. - PubMed
- Benoit-Marand M, Jaber M, Gonon F. Release and elimination of dopamine in vivo in mice lacking the dopamine transporter: functional consequences. Eur J Neurosci. 2000;12:2985–2992. - PubMed
- Chi P, Greengard P, Ryan TA. Synapsin dispersion and reclustering during synaptic activity. Nat Neurosci. 2001;4:1187–1193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases