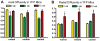Rostrocaudal analysis of corpus callosum demyelination and axon damage across disease stages refines diffusion tensor imaging correlations with pathological features - PubMed (original) (raw)
Rostrocaudal analysis of corpus callosum demyelination and axon damage across disease stages refines diffusion tensor imaging correlations with pathological features
Mingqiang Xie et al. J Neuropathol Exp Neurol. 2010 Jul.
Abstract
Noninvasive assessment of the progression of axon damage is important for evaluating disease progression and developing neuroprotective interventions in multiple sclerosis patients. We examined the cellular responses correlated with diffusion tensor imaging-derived axial (lambda(parallel)) and radial (lambda(perpendicular)) diffusivity values throughout acute (4 weeks) and chronic (12 weeks) stages of demyelination and after 6 weeks of recovery using the cuprizone demyelination of the corpus callosum model in C57BL/6 and Thy1-YFP-16 mice. The rostrocaudal progression of pathological alterations in the corpus callosum enabled spatially and temporally defined correlations of pathological features with diffusion tensor imaging measurements. During acute demyelination, microglial/macrophage activation was most extensive and axons exhibited swellings, neurofilament dephosphorylation, and reduced diameters. Axial diffusivity values decreased in the acute phase but did not correlate with axonal atrophy during chronic demyelination. In contrast, radial diffusivity increased with the progression of demyelination but did not correlate with myelin loss or astrogliosis. Unlike other animal models with progressive neurodegeneration and axon loss, the acute axon damage did not progress to discontinuity or loss of axons even after a period of chronic demyelination. Correlations of reversible axon pathology, demyelination, microglia/macrophage activation, and astrogliosis with regional axial and radial diffusivity measurements will facilitate the clinical application of diffusion tensor imaging in multiple sclerosis patients.
Figures
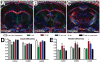
Figure 1
Diffusion tensor imaging (DTI) across rostro-caudal levels of the corpus callosum (CC) over the course of cuprizone treatment and recovery. (A–C) Color coded maps of relative anisotropy derived from DTI reflect the fiber orientation (red, medial-lateral; green, superior-inferior; blue, anterior-posterior) within white matter tracts in coronal MRI slices centered at anatomical positions within the CC to represent rostral (A; Bregma +0.75), middle (B; Bregma −0.25 mm), and caudal (C; Bregma −1.75) levels. The ROI analyzed within the CC extended from the midline to under the peak of the cingulum (outlined area). Example is after 4 weeks of cuprizone. (D, E) Quantification of axial (D) and radial (E) diffusivity values among C57BL/6 mice. One cohort of 5 mice was followed in a longitudinal series from prior to the start of treatment (pre-treatment; 8 weeks of age) and again at 4 and 12 weeks of cuprizone and after 12 weeks of cuprizone followed by a 6-week recovery period on normal chow (12 weeks cuprizone + 6 weeks). A second control cohort of 5 mice was scanned as a non-treated group at 18 weeks of age (18 weeks no cuprizone). Both axial and radial diffusivity values show a rostro-caudal pattern that is most pronounced in the caudal CC. Relative to pre-treatment controls, axial values are significantly decreased after 4 weeks of cuprizone (ap < 0.0001, bp = 0.0011, cp < 0.0001, for rostral, middle, and caudal CC, respectively) but overall exhibit a quadratic trend (p < 0.0001) with subsequent recovery to control levels. Radial diffusivity remains at pre-treatment levels through 4 weeks and then increases significantly by the 12-week time point (dp < 0.0001, ep = 0.004, and fp < 0.0001, for rostral, middle, and caudal, respectively) and remains elevated during the subsequent 6-week recovery period (gp = 0.0012, hp = 0.011, ip < 0.0001, for rostral, middle, and caudal, respectively). Pre-treatment and non-treated control cohorts exhibited similar values for each measurement. Scale bar = 1 mm.
Figure 2
Immunohistochemical analysis of myelination across rostro-caudal levels of the corpus callosum (CC) in C57BL/6 mice over the course of cuprizone treatment and recovery. (A–C) Confocal images from within sagittal brain sections immunostained for myelin oligodendrocyte glycoprotein (MOG; red; myelin marker) and NF200 (green; pan-neurofilament marker) in rostral (A), middle (B), and caudal (C) regions of the CC demonstrate a gradient in the extent of demyelination at 4 weeks of treatment. (D, E) Lower power images of coronal brain sections in the caudal region of the CC with myelin immunostained for MOG and nuclei labeled with DAPI. The normal CC area (D) expands with infiltration and amplification of cells during initial demyelination (E). Midline for each coronal section is aligned along the image border between D and E. (F) CC areas measured in low power sagittal sections of the entire CC immunostained for MOG. There was a significant increase in CC area at 4 weeks relative to all other time points (***p < 0.001). Scale bars: A = 25 μm, D = 250 μm.
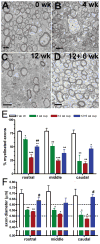
Figure 3
Ultrastructural analysis of myelin and axonal parameters throughout rostro-caudal regions of the corpus callosum (CC) over the course of cuprizone treatment in C57BL/6 mice. (A–D) Electron micrographs illustrate demyelination and subsequent remyelination during cuprizone treatment and recovery: non-treated (0 weeks) controls (A), 4 weeks (B), 12 weeks (C), and 12+6 weeks (D) of cuprizone. There is a swollen axon with disorganized cytoskeletal elements and vacuolar material at 4 weeks (center of panel B). (E) Percentages of myelinated axons in rostral, middle, and caudal regions of the CC. All regions exhibited significant decreases in percent of myelinated axons after 4 weeks of treatment; this persisted through the recovery period (*p < 0.05, **p < 0.01, or ***p < 0.001 relative to non-treated (0 weeks) controls). In the rostral CC there was a significant increase in the percent of myelinated axons during the recovery period (##p < 0.01, relative to 12 weeks cuprizone). (F) There were significant decreases in axonal diameter vs. non-treated (0 weeks) controls after 4 weeks of cuprizone, which persisted through 12 weeks of cuprizone (**p < 0.01 for rostral, *p < 0.05 for middle and caudal). After 12 weeks of cuprizone and a 6-week period on normal chow there was a significant recovery in axon diameter (#p < 0.05 for rostral and caudal vs. 12 weeks cuprizone) approaching the pre-treatment values. A–D: scale bar = 1 μm.
Figure 4
Immunohistochemical analysis of axonal damage in C57Bl/6 mice. (A–C) Double immunolabeling with NF200 (green; pan-neurofilament marker) and SMI-32 (red; non-phosphorylated neurofilament epitope) in mice treated with cuprizone for 0 weeks (A), 4 weeks (B) or 12 weeks (C) and imaged using confocal microscopy of the caudal region of the corpus callosum (CC). (D) There is significant increase in the percent of SMI-32 immunolabeled axons among the total axon population after 4 weeks in the caudal CC and in all regions after 12 weeks of cuprizone vs. non-treated (0 weeks) controls (**p < 0.01 and ***p < 0.001). (E) There was no significant difference in the density of neurofilament profiles (NF200 only + SMI-32 only + double-labeled profiles) observed throughout cuprizone treatment. Scale bar = 20 μm.
Figure 5
Axonal varicosities detected by yellow fluorescent protein (YFP) distribution in Thy1-YFP-16 mice. (A–F) Coronal cryostat sections through the caudal corpus callosum to show YFP distribution longitudinally along axons. YFP is distributed uniformly along axons prior to the start of cuprizone (A, boxed area enlarged in D). At 4 weeks of cuprizone treatment the YFP distribution along axons is non-uniform, exhibiting swellings and varicosities or beading consistent with impaired axonal transport (B, boxed area enlarged in E). At 10 weeks of treatment, YFP is more distributed throughout axons and beading appears less marked than at the 4-week time point (C, boxed area enlarged in F). (G) The extent of acute axonal damage indicated by non-uniform YFP distribution was quantified as 0 = no beading or regions of YFP loss, 1 = beading, and 2 = beading and regions of YFP loss (see methods). YFP distribution is significantly different between the 0-, 4- and 10-week time points (G, p < 0.0001). Scale bars: A–C = 240 μm, D–F = 50 μm.
Figure 6
Quantification of axial (A) and radial (B) diffusivity values in the corpus callosum (CC) of Thy1-YFP-16 mice. A cohort of 3 mice was followed in a longitudinal series from prior to the start of cuprizone treatment (0 weeks) and at 4 and 10 weeks of cuprizone treatment. Relative to controls (0 weeks), axial values are significantly decreased after 4 weeks of cuprizone (ap = 0.0006 for rostral and bp = 0.010 for caudal CC) but overall exhibit a quadratic trend (p < 0.0001) with subsequent recovery to control levels at 10 weeks (p = 0.87). In contrast, radial diffusivity is not changed until the 10 weeks cuprizone time point and is significantly increased compared to controls (cp = 0.0036, dp = 0.0001, and ep = 0.0003, for rostral, middle, and caudal, respectively).
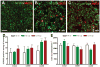
Figure 7
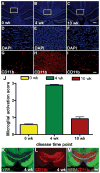
Microglia/macrophage activation in the corpus callosum (CC) of Thy1-YFP-16 mice during acute and chronic demyelination. (A–I) DAPI nuclear stain (A–F) and CD11b immunolabeling (G–I) of microglia and macrophages in coronal CC sections of mice without (A, D, G), after 4 weeks (B, E, H), and after 10 weeks (C, F, I) of cuprizone treatment. Without cuprizone, DAPI-stained nuclei are mainly aligned in rows along axons, characteristic of interfascicular oligodendrocytes (A, boxed area enlarged in D) and few cells immunolabeled by CD11b (G). After 4 weeks, DAPI staining illustrates extensive cellular infiltration (B, boxed area enlarged in E) and increased CD11b immunolabeling (H). After 10 weeks, DAPI-stained nuclei exhibit a relatively normal density but are not aligned in rows (C, boxed area enlarged in F) and CD11b immunolabeling has normalized. (J) Microglia/macrophage activation and accumulation were quantified by scoring sections on a scale of 0 to 3, with 3 as the greatest extent of CD11b-immunoreactivity (IR). CD11b-IR was significantly increased in mice fed cuprizone for 4 weeks (***p = 0.0001) or 10 weeks (***p = 0.0001) vs. non-treated control mice (0 weeks). (K–M) Coronal CC sections from Thy1-YFP-16 mice treated with cuprizone for 4 weeks with axonal YFP fluorescence (K), CD11b immunolabeling (L), and the merged image (M) to show colocalization of high CD11B-IR in areas of vesiculated axons. Scale bars: 240 μm (A–C, K–M); 50 μm (D–I).
Figure 8
Astroglial response in the corpus callosum (CC) of Thy1-YFP-16 mice during acute and chronic demyelination. (A–F) Immunolabeling for glial fibrillary acidic protein (GFAP) in coronal CC sections to identify astrocytes in mice without (A, boxed area enlarged in D), after 4 weeks (B, boxed area enlarged in E), and after 10 weeks (C, with boxed area enlarged in F) of cuprizone treatment. (G) Astrogliosis was quantified using a scoring system from 0 to 3, with 3 as the greatest extent of GFAP-immunoreactivity (IR). Astrogliosis increases from the non-treated (0 weeks) control mice to the 4-week group (***p = 0.0005) and continues to increase from 4 to 10 weeks of (**p = 0.0064) of cuprizone treatment. Scale bars: A–C = 240 μm, D–F = 50 μm.
Similar articles
- In vivo quantification of demyelination and recovery using compartment-specific diffusion MRI metrics validated by electron microscopy.
Jelescu IO, Zurek M, Winters KV, Veraart J, Rajaratnam A, Kim NS, Babb JS, Shepherd TM, Novikov DS, Kim SG, Fieremans E. Jelescu IO, et al. Neuroimage. 2016 May 15;132:104-114. doi: 10.1016/j.neuroimage.2016.02.004. Epub 2016 Feb 11. Neuroimage. 2016. PMID: 26876473 Free PMC article. - Quantitative MRI and ultrastructural examination of the cuprizone mouse model of demyelination.
Thiessen JD, Zhang Y, Zhang H, Wang L, Buist R, Del Bigio MR, Kong J, Li XM, Martin M. Thiessen JD, et al. NMR Biomed. 2013 Nov;26(11):1562-81. doi: 10.1002/nbm.2992. Epub 2013 Aug 13. NMR Biomed. 2013. PMID: 23943390 - Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum.
Sun SW, Liang HF, Trinkaus K, Cross AH, Armstrong RC, Song SK. Sun SW, et al. Magn Reson Med. 2006 Feb;55(2):302-8. doi: 10.1002/mrm.20774. Magn Reson Med. 2006. PMID: 16408263 - Oligodendrocyte lineage and subventricular zone response to traumatic axonal injury in the corpus callosum.
Sullivan GM, Mierzwa AJ, Kijpaisalratana N, Tang H, Wang Y, Song SK, Selwyn R, Armstrong RC. Sullivan GM, et al. J Neuropathol Exp Neurol. 2013 Dec;72(12):1106-25. doi: 10.1097/NEN.0000000000000009. J Neuropathol Exp Neurol. 2013. PMID: 24226267 Free PMC article. - Demyelination increases radial diffusivity in corpus callosum of mouse brain.
Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Song SK, et al. Neuroimage. 2005 May 15;26(1):132-40. doi: 10.1016/j.neuroimage.2005.01.028. Neuroimage. 2005. PMID: 15862213
Cited by
- Differentiation and quantification of inflammation, demyelination and axon injury or loss in multiple sclerosis.
Wang Y, Sun P, Wang Q, Trinkaus K, Schmidt RE, Naismith RT, Cross AH, Song SK. Wang Y, et al. Brain. 2015 May;138(Pt 5):1223-38. doi: 10.1093/brain/awv046. Epub 2015 Feb 26. Brain. 2015. PMID: 25724201 Free PMC article. - Region and dynamic specificities of adult neural stem cells and oligodendrocyte precursors in myelin regeneration in the mouse brain.
Brousse B, Magalon K, Durbec P, Cayre M. Brousse B, et al. Biol Open. 2015 Jul 3;4(8):980-92. doi: 10.1242/bio.012773. Biol Open. 2015. PMID: 26142314 Free PMC article. - Structural insights into the rodent CNS via diffusion tensor imaging.
Zhang J, Aggarwal M, Mori S. Zhang J, et al. Trends Neurosci. 2012 Jul;35(7):412-21. doi: 10.1016/j.tins.2012.04.010. Epub 2012 May 30. Trends Neurosci. 2012. PMID: 22651954 Free PMC article. Review. - Dual Mechanism of Action of Curcumin in Experimental Models of Multiple Sclerosis.
ELBini-Dhouib I, Manai M, Neili NE, Marzouki S, Sahraoui G, Ben Achour W, Zouaghi S, BenAhmed M, Doghri R, Srairi-Abid N. ELBini-Dhouib I, et al. Int J Mol Sci. 2022 Aug 4;23(15):8658. doi: 10.3390/ijms23158658. Int J Mol Sci. 2022. PMID: 35955792 Free PMC article. - Neural Stem Cells of the Subventricular Zone Contribute to Neuroprotection of the Corpus Callosum after Cuprizone-Induced Demyelination.
Butti E, Bacigaluppi M, Chaabane L, Ruffini F, Brambilla E, Berera G, Montonati C, Quattrini A, Martino G. Butti E, et al. J Neurosci. 2019 Jul 10;39(28):5481-5492. doi: 10.1523/JNEUROSCI.0227-18.2019. Epub 2019 May 28. J Neurosci. 2019. PMID: 31138656 Free PMC article.
References
- Bruck W. Inflammatory demyelination is not central to the pathogenesis of multiple sclerosis. J Neurol. 2005;252 (Suppl 5):v10–5. - PubMed
- Wilkins A, Scolding N. Protecting axons in multiple sclerosis. Mult Scler. 2008;14:1013–25. - PubMed
- Kuhlmann T, Lingfeld G, Bitsch A, et al. Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain. 2002;125:2202–12. - PubMed
- Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–85. - PubMed
- Pun S, Santos AF, Saxena S, et al. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci. 2006;9:408–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS54194/NS/NINDS NIH HHS/United States
- R01 NS054194/NS/NINDS NIH HHS/United States
- NS47592/NS/NINDS NIH HHS/United States
- NS39293/NS/NINDS NIH HHS/United States
- R01 NS039293/NS/NINDS NIH HHS/United States
- R01 NS047592/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous