TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression - PubMed (original) (raw)
Review
TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression
Li Yang et al. Trends Immunol. 2010 Jun.
Abstract
Transforming growth factor beta (TGF-beta) plays an important role in tumor initiation and progression, functioning as both a suppressor and a promoter. The mechanisms underlying this dual role of TGF-beta remain unclear. TGF-beta exerts systemic immune suppression and inhibits host immunosurveillance. Neutralizing TGF-beta enhances CD8+ T-cell- and NK-cell-mediated anti-tumor immune responses. It also increases neutrophil-attracting chemokines resulting in recruitment and activation of neutrophils with an antitumor phenotype. In addition to its systemic effects, TGF-beta regulates infiltration of inflammatory/immune cells and cancer-associated fibroblasts in the tumor microenvironment causing direct changes in tumor cells. Understanding TGF-beta regulation at the interface of tumor and host immunity should provide insights into developing effective TGF-beta antagonists and biomarkers for patient selection and efficacy of TGF-beta antagonist treatment.
Published by Elsevier Ltd.
Figures
Figure 1
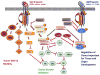
The TGF-β ligands signal through the type I and type II TGF-β receptors. Canonical signaling proceeds with phosphorylation of SMAD2 and SMAD3, which then combine with SMAD4 to enter the nucleus and mediate growth inhibition. TGF-β binding to its receptors activates many non-canonical signaling pathways, including small GTPases (RhoA, PKN, and Rock), p38 kinase and PI3 kinase pathways. These pathways are important in regulating tumor-cell migration and metastasis. In addition, bone morphogenetic proteins (BMPs) signal through SMAD1, SMAD5 or SMAD8, which form complexes with SMAD4, which activate or repress targeted gene transcription that is important for tissue and organ development. Smad 6 and 7 are negative mediators in the TGF-β signaling pathway.
Figure 2
TGF-β switches from tumor suppressor in the premalignant stages of tumorigenesis to tumor promotion in later stages of the disease leading to metastasis. Progression to metastatic disease is generally accompanied by decreased or altered TGF-β responsiveness and increased expression or activation of the TGF-β ligand. When combined with genetic or epigenetic perturbations in tumor cells, along with alteration in the tumor microenvironment, the spectrum of biological responses to TGF-β are altered. EMT: epithelial mesenchymal transition (Figure modified from Ref. 32)
Figure 3
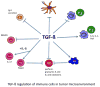
TGF-β affects multiple components of the immune system. TGF-β inhibits the function of natural killer (NK) and CD8+ CTL (cytotoxic T lymphocytes), blocking the ‘cytotoxic program’ key proteins such as perforin, granzymes and cytotoxins. TGF-β induces Treg and Th17 cell differentiation and inhibits B-cell proliferation and IgA secretion. In addition, TGF-β inhibits dendritic function, block type I macrophage and neutrophil development but promotes type II macrophages and neutrophils, and mediates the immune suppression function of MISCs.
Figure 4
TGF-β regulation of tumor microenvironment. (a) Cellular players in tumor microenvironment. (b) The mechanisms by which TGFβ signaling switches from a tumor suppressor to a tumor promoter are shown. TGFβ signals through the type II receptor mediate growth inhibition of carcinoma cells. When TβRII is deleted or downregulated, the result is increased chemokine/chemokine-receptor signaling, such as CXCL1–CXCL5/CXCR2 and SDF-1–CXCR4. Host-derived immature myeloid Gr-1+CD11b+ cells are recruited into the tumor microenvironment through these chemokine mechanisms. These Gr-1+CD11b+ cells express high level of MMPs and TGFβ1, which promote tumor invasion and immune suppression. The effect of Gr-1+CD11b+ cells on the tumor microenvironment and host immune surveillance constitute a tumor-promoting mechanism of TGFβ signaling.
Similar articles
- TGFbeta, a potent regulator of tumor microenvironment and host immune response, implication for therapy.
Yang L. Yang L. Curr Mol Med. 2010 Jun;10(4):374-80. doi: 10.2174/156652410791317039. Curr Mol Med. 2010. PMID: 20455854 Review. - Human tumour immune evasion via TGF-β blocks NK cell activation but not survival allowing therapeutic restoration of anti-tumour activity.
Wilson EB, El-Jawhari JJ, Neilson AL, Hall GD, Melcher AA, Meade JL, Cook GP. Wilson EB, et al. PLoS One. 2011;6(9):e22842. doi: 10.1371/journal.pone.0022842. Epub 2011 Sep 6. PLoS One. 2011. PMID: 21909397 Free PMC article. - TGF-β regulated leukemia cell susceptibility against NK targeting through the down-regulation of the CD48 expression.
Huang CH, Liao YJ, Chiou TJ, Huang HT, Lin YH, Twu YC. Huang CH, et al. Immunobiology. 2019 Sep;224(5):649-658. doi: 10.1016/j.imbio.2019.07.002. Epub 2019 Aug 9. Immunobiology. 2019. PMID: 31421859 - Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity.
Kopp HG, Placke T, Salih HR. Kopp HG, et al. Cancer Res. 2009 Oct 1;69(19):7775-83. doi: 10.1158/0008-5472.CAN-09-2123. Epub 2009 Sep 8. Cancer Res. 2009. PMID: 19738039 - TGF-β Family Signaling in Tumor Suppression and Cancer Progression.
Seoane J, Gomis RR. Seoane J, et al. Cold Spring Harb Perspect Biol. 2017 Dec 1;9(12):a022277. doi: 10.1101/cshperspect.a022277. Cold Spring Harb Perspect Biol. 2017. PMID: 28246180 Free PMC article. Review.
Cited by
- Identification of gastric cancer subtypes based on pathway clustering.
Li L, Wang X. Li L, et al. NPJ Precis Oncol. 2021 Jun 2;5(1):46. doi: 10.1038/s41698-021-00186-z. NPJ Precis Oncol. 2021. PMID: 34079012 Free PMC article. - The return of Dr Jekyll in cancer metastasis.
Attisano L, Wrana JL. Attisano L, et al. EMBO J. 2012 Dec 12;31(24):4486-7. doi: 10.1038/emboj.2012.316. Epub 2012 Nov 27. EMBO J. 2012. PMID: 23188084 Free PMC article. - The Fyn-STAT5 Pathway: A New Frontier in IgE- and IgG-Mediated Mast Cell Signaling.
Pullen NA, Falanga YT, Morales JK, Ryan JJ. Pullen NA, et al. Front Immunol. 2012 May 11;3:117. doi: 10.3389/fimmu.2012.00117. eCollection 2012. Front Immunol. 2012. PMID: 22593761 Free PMC article. - The TGFβ type I receptor kinase inhibitor vactosertib in combination with pomalidomide in relapsed/refractory multiple myeloma: a phase 1b trial.
Malek E, Rana PS, Swamydas M, Daunov M, Miyagi M, Murphy E, Ignatz-Hoover JJ, Metheny L, Kim SJ, Driscoll JJ. Malek E, et al. Nat Commun. 2024 Aug 27;15(1):7388. doi: 10.1038/s41467-024-51442-2. Nat Commun. 2024. PMID: 39191755 Free PMC article. Clinical Trial. - SNX4 Is Correlated With Immune Infiltration and Prognosis in Clear Cell Renal Cell Carcinoma.
Chai YM, Zhou ZB, Liu RZ, Cui YS, Zhang Y. Chai YM, et al. World J Oncol. 2024 Oct;15(5):809-824. doi: 10.14740/wjon1868. Epub 2024 Jul 18. World J Oncol. 2024. PMID: 39328330 Free PMC article.
References
- Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6 (7):506–520. - PubMed
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425 (6958):577–584. - PubMed
- Nakao A, et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389 (6651):631–635. - PubMed
- Hayashi H, et al. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell. 1997;89 (7):1165–1173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials