Marine molecular machines: heterocyclization in cyanobactin biosynthesis - PubMed (original) (raw)
Marine molecular machines: heterocyclization in cyanobactin biosynthesis
John A McIntosh et al. Chembiochem. 2010.
Abstract
Natural products that contain amino-acid-derived (Cys, Ser, Thr) heterocycles are ubiquitous in nature, yet key aspects of their biosynthesis remain undefined. Cyanobactins are heterocyclic ribosomal peptide natural products from cyanobacteria, including symbiotic bacteria living with marine ascidians. In contrast to other ribosomal peptide heterocyclases that have been studied, the cyanobactin heterocyclase is a single protein that does not require an oxidase enzyme. Using this simplifying condition, we provide new evidence to support the hypothesis that these enzymes are molecular machines that use ATP in a product binding or orientation cycle. Further, we show that both protease inhibitors and ATP analogues inhibit heterocyclization and define the order of biochemical steps in the cyanobactin biosynthetic pathway. The cyanobactin pathway enzymes, PatD and TruD, are thiazoline and oxazoline synthetases.
Figures
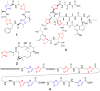
Figure 1
Heterocyclic natural products. Shown are patellamide C (1), epothilone B (2), thiostrepton (3), and microcin B17 (4).
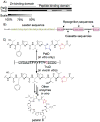
Figure 2
A) Shown are alignments between PatD and TruD. Darker regions indicate regions of higher identity. B) Sequence of TruE2 precursor peptide is shown, with naturally heterocyclized residues highlighted in red. C) A zoomed-in view of the C-terminal cassette in TruE2. In vitro, PatD modifies one Thr and one Cys in this cassette, while TruD modifies one Cys both in vitro and in vivo. In nature, in combination with other biosynthetic enzymes the TruD product shown is converted to the prenylated, heterocyclic natural product patellin 6.
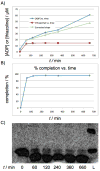
Figure 3
Stoichiometry of heterocycle formation. A) rates of ADP formation and thiazoline synthesis are overlaid; corrected slope denotes the rate of ATP hydrolysis when corrected for the background hydrolysis. B) %-completion of the heterocyclization reaction as determined by SDS-PAGE gel densitometry. C) SDS-PAGE gel used to derive [thiazoline] and %-completion.
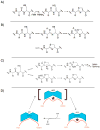
Scheme 1
Shown above are mechanistic possibilities for heterocyclization. A) oxidation preceding heterocycle formation B) activation of the adjacent carbonyl oxygen, perhaps with phosphate from ATP C) intein mechanism for thiazoline formation as a side-product D) molecular machine mechanism for heterocyclase enzymes.
Similar articles
- Insights into the Mechanism of the Cyanobactin Heterocyclase Enzyme.
Ge Y, Czekster CM, Miller OK, Botting CH, Schwarz-Linek U, Naismith JH. Ge Y, et al. Biochemistry. 2019 Apr 23;58(16):2125-2132. doi: 10.1021/acs.biochem.9b00084. Epub 2019 Apr 5. Biochemistry. 2019. PMID: 30912640 Free PMC article. - Insights into heterocyclization from two highly similar enzymes.
McIntosh JA, Donia MS, Schmidt EW. McIntosh JA, et al. J Am Chem Soc. 2010 Mar 31;132(12):4089-91. doi: 10.1021/ja9107116. J Am Chem Soc. 2010. PMID: 20210311 Free PMC article. - Cyanobactins-ribosomal cyclic peptides produced by cyanobacteria.
Sivonen K, Leikoski N, Fewer DP, Jokela J. Sivonen K, et al. Appl Microbiol Biotechnol. 2010 May;86(5):1213-25. doi: 10.1007/s00253-010-2482-x. Epub 2010 Feb 27. Appl Microbiol Biotechnol. 2010. PMID: 20195859 Free PMC article. Review. - Roads to Rome: Role of Multiple Cassettes in Cyanobactin RiPP Biosynthesis.
Gu W, Sardar D, Pierce E, Schmidt EW. Gu W, et al. J Am Chem Soc. 2018 Nov 28;140(47):16213-16221. doi: 10.1021/jacs.8b09328. Epub 2018 Nov 14. J Am Chem Soc. 2018. PMID: 30387998 Free PMC article. - The Biochemistry and Structural Biology of Cyanobactin Pathways: Enabling Combinatorial Biosynthesis.
Gu W, Dong SH, Sarkar S, Nair SK, Schmidt EW. Gu W, et al. Methods Enzymol. 2018;604:113-163. doi: 10.1016/bs.mie.2018.03.002. Epub 2018 May 4. Methods Enzymol. 2018. PMID: 29779651 Free PMC article. Review.
Cited by
- Micrococcin cysteine-to-thiazole conversion through transient interactions between the scaffolding protein TclI and the modification enzymes TclJ and TclN.
Calvopina-Chavez DG, Bursey DM, Tseng Y-J, Patil LM, Bewley KD, Bennallack PR, McPhie JM, Wagstaff KB, Daley A, Miller SM, Moody JD, Price JC, Griffitts JS. Calvopina-Chavez DG, et al. Appl Environ Microbiol. 2024 Jun 18;90(6):e0024424. doi: 10.1128/aem.00244-24. Epub 2024 May 23. Appl Environ Microbiol. 2024. PMID: 38780510 Free PMC article. - Chemoenzymatic Late-Stage Modifications Enable Downstream Click-Mediated Fluorescent Tagging of Peptides.
Colombano A, Dalponte L, Dall'Angelo S, Clemente C, Idress M, Ghazal A, Houssen WE. Colombano A, et al. Angew Chem Int Ed Engl. 2023 Apr 11;62(16):e202215979. doi: 10.1002/anie.202215979. Epub 2023 Mar 10. Angew Chem Int Ed Engl. 2023. PMID: 36815722 Free PMC article. - Biochemical characterization of a cyanobactin arginine-_N_-prenylase from the autumnalamide biosynthetic pathway.
Clemente C, Johnson N, Ouyang X, Popin RV, Dall'Angelo S, Wahlsten M, Jokela J, Colombano A, Nardone B, Fewer DP, Houssen WE. Clemente C, et al. Chem Commun (Camb). 2022 Oct 27;58(86):12054-12057. doi: 10.1039/d2cc01799g. Chem Commun (Camb). 2022. PMID: 36193595 Free PMC article. - Control of Nucleophile Chemoselectivity in Cyanobactin YcaO Heterocyclases PatD and TruD.
Gu W, Zheng Y, Pogorelov T, Nair SK, Schmidt EW. Gu W, et al. ACS Chem Biol. 2022 May 20;17(5):1215-1225. doi: 10.1021/acschembio.2c00147. Epub 2022 Apr 14. ACS Chem Biol. 2022. PMID: 35420020 Free PMC article. - A roadmap for metagenomic enzyme discovery.
Robinson SL, Piel J, Sunagawa S. Robinson SL, et al. Nat Prod Rep. 2021 Nov 17;38(11):1994-2023. doi: 10.1039/d1np00006c. Nat Prod Rep. 2021. PMID: 34821235 Free PMC article. Review.
References
- Schmidt EW. The UCSD Guardian. 1991;74
- Lee FY, Borzilleri R, Fairchild CR, Kim SH, Long BH, Reventos-Suarez C, Vite GD, Rose WC, Kramer RA, Kelly WL, Hillson NJ, Walsh CT. Clin Cancer Res. 2001;7:1429. - PubMed
- Roy RS, Gehring AM, Milne JC, Belshaw PJ, Walsh CT. Nat Prod Rep. 1999;16:249. - PubMed
- Anderson B, Hodgkin D, Viswamitra MA. Nature. 1970;225:233. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources