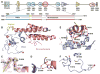Pro isomerization in MLL1 PHD3-bromo cassette connects H3K4me readout to CyP33 and HDAC-mediated repression - PubMed (original) (raw)
Pro isomerization in MLL1 PHD3-bromo cassette connects H3K4me readout to CyP33 and HDAC-mediated repression
Zhanxin Wang et al. Cell. 2010.
Abstract
The MLL1 gene is a frequent target for recurrent chromosomal translocations, resulting in transformation of hematopoietic precursors into leukemia stem cells. Here, we report on structure-function studies that elucidate molecular events in MLL1 binding of histone H3K4me3/2 marks and recruitment of the cyclophilin CyP33. CyP33 contains a PPIase and a RRM domain and regulates MLL1 function through HDAC recruitment. We find that the PPIase domain of CyP33 regulates the conformation of MLL1 through proline isomerization within the PHD3-Bromo linker, thereby disrupting the PHD3-Bromo interface and facilitating binding of the MLL1-PHD3 domain to the CyP33-RRM domain. H3K4me3/2 and CyP33-RRM target different surfaces of MLL1-PHD3 and can bind simultaneously to form a ternary complex. Furthermore, the MLL1-CyP33 interaction is required for repression of HOXA9 and HOXC8 genes in vivo. Our results highlight the role of PHD3-Bromo cassette as a regulatory platform, orchestrating MLL1 binding of H3K4me3/2 marks and cyclophilin-mediated repression through HDAC recruitment.
Figures
Figure 1. Construct Design and Crystal Structure of MLL1 PHD3-Bromo Cassette in the Free State
(A) Domain architecture of MLL1 is shown in the top panel. Two coexpression constructs of MLL1 PHD3-Bromo cassette used for crystallization are shown in the bottom panel. (B) A ribbon view of the crystal structure of MLL1 PHD3-Bromo cassette with labeling of secondary structure elements. Pocket residues within PHD3 involved in Kme recognition are shown in stick representation, together with residues involved in direct interaction at the interface of the PHD3-Bromo cassette. (C) An expanded view highlighting the cis peptide bond at Pro1629 in the linker segment connecting MLL1 PHD3 and Bromo domains. Residues 1628–1630 are shown in stick representation, with the corresponding 2Fo-Fc electron density contoured at 1σ. (D) An expanded view of the interdomain interaction between Glu1605 from PHD3 and the Val1723-Leu1724 backbone amides from Bromo. (E) An expanded view highlighting the interfacial hydrophobic and hydrogen bonding interactions between PHD3 (in blue) and Bromo (in salmon). (F) The partially formed Kme recognition pocket on the surface of PHD3. The Tyr1581 residue, which is not part of the aromatic-lined pocket, is indicated by a red asterisk. See also Figure S1.
Figure 2. Crystal Structure of MLL1 PHD3-Bromo Cassette Bound to H3K4me3/2-Containing Peptides, Together with Binding Data on Wild-Type and Mutant PHD3
(A) ITC binding curves of MLL1 PHD3-Bromo cassette with H3K4me-containing peptides. (B) A ribbon view of the crystal structure of MLL1 PHD3-Bromo cassette in complex with H3(1-9)K4me3 peptide. _cis_-configured Pro1629 and pocket residues for trimethyl-lysine recognition are labeled accordingly. (C) A view of the hydrogen bonding interactions between MLL1 PHD3 (blue) and bound H3K4me3 peptide (yellow) in the crystal structure of the complex. (D) ITC profiles for the binding of H3(1-15)K4me3 peptide to wild-type (WT) MLL1 PHD3-Bromo and its W1594E, Y1581A, and Q1587A mutants. (E) A view of the superimposed structures of the K4me3 binding pocket of MLL1 PHD3 in the free (silver) and H3K4me3-bound (blue) states. The blue and red arrows highlight the conformational changes in the loop spanning Tyr1576-Met1585 segment and involving Tyr1581, respectively, on complex formation. (F) A view of the superimposed structures of the peptide N terminus binding pocket of MLL1 PHD3 in the free (silver) and H3K4me3-bound (blue) states. The Pro1614-Val1617 segment adopts an α helix (silver) in the free state but changes into a turn fold (blue) in the bound state. (G) Surface electrostatic view of MLL1 PHD3 with bound H3(1-6)K4me3 peptide in a space-filling representation. Note that access is blocked to both the trime-thylammonium group of H3K4me3 (indicated by red asterisk) and the N terminus (indicated by red pound symbol) in this complex. (H) ITC profiles for the binding of MLL1 PHD3-Bromo to wild-type and two modified H3K4me3 peptides containing one or three additional Ala residues added to the N terminus. See also Figure S2.
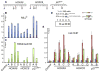
Figure 3. MLL1 PHD3 Is Important for Stable Target Gene Binding In Vivo
(A) Positions of primer sets used for real-time PCR. (B and C) ChIP analysis with antibodies for MLLC (B) or H3K4me3 (normalized to H3 levels) (C) at the target genes HOXA9, HOXC8, and p27KIP1. ChIP results shown are typical for at least three independent experiments. Error bars represent standard deviation of three separate PCR reactions. (D) Four separate stable cell lines were generated expressing the MLLN fragments fh-MLLCXXC-PHD3 and fh-MLLCXXC-PHD3 (W1594E) (W1594E point mutation in MLL1 PHD3). (E) ChIP signal with an HA antibody for the stable cell lines in (D) at MLL1 target genes. ChIP signal was calculated for each cell line individually, and then the results for 2 and 3 were averaged together, as were the results for 4 and 5. Averages were the results of three independent experiments for each cell line. Error bars represent standard deviation across the averaged values.
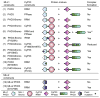
Figure 4. Complex Formation Results between Different Combinations of Constructs of MLL1/2 PHD3-Bromo Cassette and CyP33
The # symbol indicates that complex was formed between PHD3-Bromo and RRM by coexpression of vectors of PHD3-Bromo, RRM, and PPIase together in E. coli. See also Figure S3.
Figure 5. NMR-Based Mapping of Interacting Surfaces and Solution Structure Defining the Interface between MLL1 PHD3 and CyP33 RRM
(A) Crystal structure of MLL1 PHD3 (1566–1628) with labeling of secondary structure elements. Residues exhibiting large chemical shift perturbations (Δδave > 0.15 ppm) upon interacting with unlabeled CyP33 RRM are colored in yellow. Δδave = [(Δδ2HN+(ΔδN/5)2)/2]1/2. (B) Crystal structure of CyP33 RRM (5–82) with labeling of secondary structure elements. Residues exhibiting large chemical shift perturbations (Δδave > 0.15 ppm) upon interacting with unlabeled MLL1 PHD3 are colored in yellow. br>(C) The backbone trace of 20 NMR refined structures of the complex containing the MLL1 PHD3 fragment (1603–1619, blue) and CyP33 RRM (2–82, magenta). (D) An alternate view of the NMR solution structure of the complex between a fragment of MLL1 PHD3 (1603–1619, blue) and the CyP33 RRM (2–82, magenta). (E) A ribbon representation of the interaction between MLL1 PHD3 fragment (1603–1619, blue) and CyP33 RRM (2–82, magenta) based on the NMR structure of the complex. Residues involved in the interaction between MLL1 PHD3 and CyP33 RRM are colored in green and yellow, respectively. (F) Spectral overlap of the selected regions of 1H,13C-HSQC of MLL1 (1566–1639; segment contains entire PHD3 finger, the linker, and a short segment of the αZ helix of Bromo) in the free state (black) and bound to CyP33 RRM (1–82) (red). (G) Model of the complex between MLL1 PHD3 (1566–1628, blue) and the CyP33 RRM (2–82, magenta) based on the structure in (D) after superimposing of their respective PHD3 α2 helices. (H) ITC curves for binding of H3(1-15)K4me3/2 peptide to the complex of MLL1 PHD3-Bromo and full-length CyP33. (I) Complexation shifts in Trp1594 indole resonance (black cross peak) in the 1H,15N-HSQC spectrum of MLL1 PHD3 on sequential addition of H3K4me3 (green cross peak), and CyP33 RRM (red cross peak). See also Figure S4.
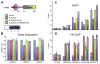
Figure 6. Mutations in Either RRM or PPIase Domains Affect CyP33-Regulated Gene Expression and Histone Deacetylation
(A) 293T cells were transiently transfected with an empty vector (1, blue), wild-type full-length FLAG and HA tagged CyP33 (2, red) or full-length CyP33 that has either the F51D (3, green) or F196E/M197E (4, purple) point mutations. A western blot with an anti-FLAG antibody indicates that all three constructs were expressed at high levels. (B) Real-time PCR quantification of gene expression levels from the transfected cells in (A) shows that HOXA9 and HOXC8 expression were reduced in the presence of wild-type CyP33 (red bars) but not in cells expressing the F51D (green bars)or F196E/M197E (purple bars) point mutations or the empty vector control (blue bars). GAPDH expression levels were unaffected by expression of any constructs. Error bars represent the standard deviation of two independent experiments. (C and D) ChIP experiments with an anti-H3 pan acetyl (AcH3) antibody (C), and an anti-HA antibody (D) at HOXA9, HOXC8, and at a control region. PCR primer/probe sets are as indicated in Figure 3. ChIP results shown are typical for at least three independent experiments. Error bars represent standard deviation of three separate PCR reactions. (C) ChIP shows decreased H3 acetylation levels at HOXA9 and HOXC8 in wild-type CyP33-expressing cells (red bars) but not in cells expressing the F51D (green bars) and F196E/M197E (purple bars) point mutations or the empty vector control (blue bars) (D) Anti-HA ChIP shows that wild-type (red bars) and F196E/M197E (purple bars) versions of CyP33 bound to HOXA9 and HOXC8, while the F51D point mutation exhibited variably reduced CyP33 binding. See also Figure S5.
Figure 7. Schematic Outlining the Proposed Regulation of MLL1 Function by Cyclophilin-Mediated Proline Isomerization within PHD3-Bromo Cassette
(A) A scheme showing transitions between two states of the MLL1 complex mediated by CyP33. CyP33 binding to MLL1 increases the recruitment of core-pressors to the repression domain, switching the balance of MLL1 functions from activation to repression of target genes. It should be noted that involvement of the RNA component in the scheme needs validation. (B) Structure of MLL1 PHD3-Bromo cassette with _cis_-configured Pro1629 (left) and model of MLL1 PHD3-Bromo-CyP33 RRM complex with _trans_-configured Pro1629 (right).
Comment in
- Flipping MLL1's switch one proline at a time.
Grow EJ, Wysocka J. Grow EJ, et al. Cell. 2010 Jun 25;141(7):1108-10. doi: 10.1016/j.cell.2010.06.013. Cell. 2010. PMID: 20602992 - Leukaemia: MLL makes friends and influences.
McCarthy N. McCarthy N. Nat Rev Cancer. 2010 Aug;10(8):529. doi: 10.1038/nrc2904. Nat Rev Cancer. 2010. PMID: 20677349 No abstract available.
Similar articles
- Cyp33 binds AU-rich RNA motifs via an extended interface that competitively disrupts the gene repressive Cyp33-MLL1 interaction in vitro.
Lloyd NR, Wuttke DS. Lloyd NR, et al. PLoS One. 2021 Feb 19;16(2):e0237956. doi: 10.1371/journal.pone.0237956. eCollection 2021. PLoS One. 2021. PMID: 33606679 Free PMC article. - Molecular mechanism of MLL PHD3 and RNA recognition by the Cyp33 RRM domain.
Hom RA, Chang PY, Roy S, Musselman CA, Glass KC, Selezneva AI, Gozani O, Ismagilov RF, Cleary ML, Kutateladze TG. Hom RA, et al. J Mol Biol. 2010 Jul 9;400(2):145-54. doi: 10.1016/j.jmb.2010.04.067. Epub 2010 May 8. J Mol Biol. 2010. PMID: 20460131 Free PMC article. - The PHD3 domain of MLL acts as a CYP33-regulated switch between MLL-mediated activation and repression.
Park S, Osmers U, Raman G, Schwantes RH, Diaz MO, Bushweller JH. Park S, et al. Biochemistry. 2010 Aug 10;49(31):6576-86. doi: 10.1021/bi1009387. Biochemistry. 2010. PMID: 20677832 Free PMC article. - Mixed lineage leukemia: a structure-function perspective of the MLL1 protein.
Cosgrove MS, Patel A. Cosgrove MS, et al. FEBS J. 2010 Apr;277(8):1832-42. doi: 10.1111/j.1742-4658.2010.07609.x. Epub 2010 Mar 4. FEBS J. 2010. PMID: 20236310 Free PMC article. Review. - Targeting protein-protein interaction between MLL1 and reciprocal proteins for leukemia therapy.
Wang ZH, Li DD, Chen WL, You QD, Guo XK. Wang ZH, et al. Bioorg Med Chem. 2018 Jan 15;26(2):356-365. doi: 10.1016/j.bmc.2017.11.045. Epub 2017 Dec 1. Bioorg Med Chem. 2018. PMID: 29254892 Review.
Cited by
- Small-molecule modulators of methyl-lysine binding for the CBX7 chromodomain.
Ren C, Morohashi K, Plotnikov AN, Jakoncic J, Smith SG, Li J, Zeng L, Rodriguez Y, Stojanoff V, Walsh M, Zhou MM. Ren C, et al. Chem Biol. 2015 Feb 19;22(2):161-8. doi: 10.1016/j.chembiol.2014.11.021. Epub 2015 Feb 5. Chem Biol. 2015. PMID: 25660273 Free PMC article. - Hijacked in cancer: the KMT2 (MLL) family of methyltransferases.
Rao RC, Dou Y. Rao RC, et al. Nat Rev Cancer. 2015 Jun;15(6):334-46. doi: 10.1038/nrc3929. Nat Rev Cancer. 2015. PMID: 25998713 Free PMC article. Review. - The co-chaperones Fkbp4/5 control Argonaute2 expression and facilitate RISC assembly.
Martinez NJ, Chang HM, Borrajo Jde R, Gregory RI. Martinez NJ, et al. RNA. 2013 Nov;19(11):1583-93. doi: 10.1261/rna.040790.113. Epub 2013 Sep 18. RNA. 2013. PMID: 24049110 Free PMC article. - Structural basis for regulation of the Crk signaling protein by a proline switch.
Sarkar P, Saleh T, Tzeng SR, Birge RB, Kalodimos CG. Sarkar P, et al. Nat Chem Biol. 2011 Jan;7(1):51-7. doi: 10.1038/nchembio.494. Epub 2010 Dec 5. Nat Chem Biol. 2011. PMID: 21131971 Free PMC article. - Endothelial EGLN3-PKM2 signaling induces the formation of acute astrocytic barrier to alleviate immune cell infiltration after subarachnoid hemorrhage.
Duan M, Ru X, Zhou J, Li Y, Guo P, Kang W, Li W, Chen Z, Feng H, Chen Y. Duan M, et al. Fluids Barriers CNS. 2024 May 16;21(1):42. doi: 10.1186/s12987-024-00550-8. Fluids Barriers CNS. 2024. PMID: 38755642 Free PMC article.
References
- Andreotti AH. Native state proline isomerization: an intrinsic molecular switch. Biochemistry. 2003;42:9515–9524. - PubMed
- Ayton PM, Cleary ML. Molecular mechanisms of leukemogenesis mediated by MLL fusion proteins. Oncogene. 2001;20:5695–5707. - PubMed
- Cléry A, Blatter M, Allain FH. RNA recognition motifs: boring? Not quite. Curr Opin Struct Biol. 2008;18:290–298. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases