Are two better than one? Analysis of an FtsK/Xer recombination system that uses a single recombinase - PubMed (original) (raw)
Are two better than one? Analysis of an FtsK/Xer recombination system that uses a single recombinase
Sophie Nolivos et al. Nucleic Acids Res. 2010 Oct.
Abstract
Bacteria harbouring circular chromosomes have a Xer site-specific recombination system that resolves chromosome dimers at division. In Escherichia coli, the activity of the XerCD/dif system is controlled and coupled with cell division by the FtsK DNA translocase. Most Xer systems, as XerCD/dif, include two different recombinases. However, some, as the Lactococcus lactis XerS/dif(SL) system, include only one recombinase. We investigated the functional effects of this difference by studying the XerS/dif(SL) system. XerS bound and recombined dif(SL) sites in vitro, both activities displaying asymmetric characteristics. Resolution of chromosome dimers by XerS/dif(SL) required translocation by division septum-borne FtsK. The translocase domain of L. lactis FtsK supported recombination by XerCD/dif, just as E. coli FtsK supports recombination by XerS/dif(SL). Thus, the FtsK-dependent coupling of chromosome segregation with cell division extends to non-rod-shaped bacteria and outside the phylum Proteobacteria. Both the XerCD/dif and XerS/dif(SL) recombination systems require the control activities of the FtsKγ subdomain. However, FtsKγ activates recombination through different mechanisms in these two Xer systems. We show that FtsKγ alone activates XerCD/dif recombination. In contrast, both FtsKγ and the translocation motor are required to activate XerS/dif(SL) recombination. These findings have implications for the mechanisms by which FtsK activates recombination.
Figures
Figure 1.
The FtsK-XerCD/dif and FtsK-XerS/difSL systems. (A) Left: diagram of the XerC, XerD, XerS and Cre proteins, with their length and domain organization (in amino acid). The conserved residues involved in catalysis are indicated. Right: difSL and dif sites of representative bacteria with the recombinase binding sites, separated by the central region (CR) indicated. In difSL sites, upper case bases are part of the previously defined minimal site (28) and bases shown in bold typeface are inverted repeats within this minimal site. The left-half site is indicated by the pale grey bar and the right-half site by the dark grey bar. This convention is used throughout the paper. In dif sites, the two half sites recognized by XerC and XerD, respectively, are indicated. Lla, L. lactis; Spn, Streptococcus pneumoniae; Spy, S. pyogenes; Sag, S. agalactiae; Eco, E. coli; Hin, H. influenzae; Vch, V. cholerae; Ngo, Neisseria gonorrhoeae; Bsub, B. subtilis. (B) A diagram of the mechanism of recombination by Y-recombinase based on the data obtained for the Cre/loxP system. Y indicates the catalytic tyrosine residue. OH is the 5′ hydroxyl group created by DNA cleavages.
Figure 2.
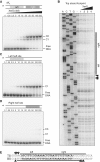
Asymmetric binding of XerS to difSL. (A) Titration of a 142-bp DNA fragment containing difSL by increasing concentrations of XerS in an EMSA experiment. The DNA substrate is shown at the top. XerS concentrations are given in nano molar and the positions of the free DNA probes and the C1, and C2 XerS/DNA complexes are indicated. The asterisk indicates the 5′-labelled strand. (B) DNaseI footprinting of the top strand (TS) of difSL. Experiments were carried out with increasing XerS concentrations, as described in the ‘Experimental procedures’ section. A, C, T and G, ladder sequence of the _difSL_-containing substrate; (−) lane, no XerS; Lanes 1, 2, 3 and 4: reactions contained 12.5, 25, 50 and 100 nM XerS, respectively. The region protected from cleavage is indicated by the black bars and positions of increased cleavage by arrows. (C and D) Same experiments as in (A), with substrates containing mutated right- and left-half sites, respectively. (E) Summary of the data for the difSL sequence. The footprint of the BS is shown in
Supplementary Figure S2
. Black bars, protected regions; Arrows, positions hypersensitive to cleavage.
Figure 3.
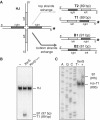
Strand exchange by XerS. (A) Left: diagram of the HJ substrate with the labelled strand indicated by asterisk. The left and right difSL half sites are indicated by pale grey and dark grey, respectively. Right: resolution products (T1, T2 and B1, B2; with their respective sizes indicated) obtained by exchange of either the top or bottom pairs of strands (defined in Figure 2). Labelled strands are indicated by asterisk. (B) PAGE analysis of the recombination products with positions of the substrate (HJ), B1 and T1 products indicated. (−) lane, no XerS; XerS and XerSY341F lanes, reactions contained 200 mM of the protein indicated. (C) Analysis of the individual DNA strands by denaturing PAGE. Positions of the labelled strands are indicated. (AGCT) lanes, size ladder (‘Experimental procedures’ section). (−) lane, no XerS; (+) lane: reaction contained 200 nM XerS.
Figure 4.
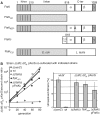
Resolution of chromosome dimers by XerS/difSL. (A) Diagram of the FtsK mutants used. Top line, wt E. coli FtsK with the three domains and the C-terminal subdomains indicated (α,β,γ). Black bars represent the A and B Walker-type motifs. Co-ordinates at the top are in amino acid. The grey bar labelled (A) indicates the mutation of the FtsKATP- mutant. The FtsKC domain was produced from a plasmid (pFtsKC; ‘Experimental procedures’ section) under the control of the pBAD promoter and was used in Δ(ftsKC) strains in the presence of 0.025% arabinose. (B) Left: typical co-culture experiments. A Δ(dif)::difSL strain carrying the pXerS plasmid was co-cultured with strains carrying either the dif site [Δ(xerC) and wt strains] or the difSL site in place of dif, the pXerS plasmid and the indicated ftsK allele [Δ(ftsKC), _ftsKATP_- and Δ(ftsKC) pFtsKC strains]. Right: frequencies of unresolved dimers in the indicated strains, calculated from experiments in B (34). The mean results of at least three independent experiments with standard deviations are shown.
Figure 5.
XerS/difSL recombination depends on the translocation activity of FtsKC. (A) The recombination reporter cassettes used consist of two directly repeated difSL, separated by the lacI gene, inserted at the natural position of dif. Recombination deletes lacI, giving rise to blue colonies on indicator medium. An example of assessments made by plating is shown. (B) Recombination frequencies in strains carrying the difSL-lacI-difSL cassette and the indicated ftsK alleles (‘Experimental procedures’ section). Proteins encoded by the Δ(ftsKC) and _ftsKATP_− mutations and the pFtsKC plasmid are shown in Figure 4A. Strains containing pFtsKC were grown in absence of inducer (NI) or in presence (I) of 0.025% arabinose.
Figure 6.
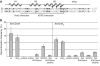
FtsK must reach difSL to induce recombination. (A) Alignment of the FtsKγ subdomains of E. coli (Ec) and L. lactis (Ll). Structural features of the Ec FtsKγ are indicated: the H1-3 helices and the wing of the w-helix DNA-binding domain. Identical residues are shown in bold and residues for which mutation leads to the KOPS-blind phenotype are indicated by stars. The regions thought to interact with XerD and the KOPS motif are indicated. The co-ordinate of the last residue aligned here is given to the right. (B) Recombination frequencies in strain carrying either a dif-lacI-dif (left, XerCD/dif) or a difSL-lacI-difSL (right, XerS/difSL) recombination cassette, the pXerC or pXerS plasmids, respectively, and the indicated ftsK alleles and/or insertion of non-permissive KOPS motifs close to one of the recombination sites (3KOPSi; ‘Experimental procedures’ section). The Δ(ftsKC)::ftsKCLl allele carries the whole C-terminal domain of L. lactis FtsK in place of the E. coli domain (‘Experimental procedures’ section; Figure 4A). The ftsKKOPSblind allele carries mutations of the three residues indicated by stars in (A).
Figure 7.
FtsKγ induces XerCD/dif but not XerS/difSL recombination. (A) Alignment of the FtsK interaction region of XerD with its corresponding region in XerS. Residues inferred to interact with FtsK are indicated and shown in bold typeface [R1300A, E1303A, E1306A; (21)]. The co-ordinate of the last residue is given to the right. (B) Western-blot analysis of FtsKγ production. Cells were collected from the indicated strains during the course of the experiments shown in (C). His-Flag-tagged E. coli and L. lactis FtsKγ were detected with an anti-FLAG antibody (‘Experimental procedures’ section). M, Purified E. coli His-FLAG-FtsKγ, with its size indicated. The faint bands that migrate faster than the FtsKγ peptides are background detection in the migration front of the gel. (C) Recombination frequencies in strains carrying either a dif-lacI-dif (left, XerCD/dif) or a difSL-lacI-difSL (right, XerS/difSL) recombination cassette and the indicated ftsK alleles and FtsKγ-coding plasmid. Recombination was induced by transformation with the pXerC plasmid (all strains in the XerCD/dif panel except strains labelled pXerCD for which the pXerC-XerD plasmid was used; ‘Experimental procedures’ section), or the pXerS plasmids (XerS/difSL panel). NI, absence of inducer; I, FtsKγ production was induced by adding 0.1% arabinose. The dotted lines and grey zones indicate the FtsKC-independent recombination background for the two systems. In both cases, the recombination background in the absence of the cognate recombinase is below 10−3 (data not shown).
Similar articles
- The unconventional Xer recombination machinery of Streptococci/Lactococci.
Le Bourgeois P, Bugarel M, Campo N, Daveran-Mingot ML, Labonté J, Lanfranchi D, Lautier T, Pagès C, Ritzenthaler P. Le Bourgeois P, et al. PLoS Genet. 2007 Jul;3(7):e117. doi: 10.1371/journal.pgen.0030117. PLoS Genet. 2007. PMID: 17630835 Free PMC article. - FtsK translocation on DNA stops at XerCD-dif.
Graham JE, Sivanathan V, Sherratt DJ, Arciszewska LK. Graham JE, et al. Nucleic Acids Res. 2010 Jan;38(1):72-81. doi: 10.1093/nar/gkp843. Epub 2009 Oct 23. Nucleic Acids Res. 2010. PMID: 19854947 Free PMC article. - Species specificity in the activation of Xer recombination at dif by FtsK.
Yates J, Aroyo M, Sherratt DJ, Barre FX. Yates J, et al. Mol Microbiol. 2003 Jul;49(1):241-9. doi: 10.1046/j.1365-2958.2003.03574.x. Mol Microbiol. 2003. PMID: 12823825 - Xer Site Specific Recombination: Double and Single Recombinase Systems.
Castillo F, Benmohamed A, Szatmari G. Castillo F, et al. Front Microbiol. 2017 Mar 20;8:453. doi: 10.3389/fmicb.2017.00453. eCollection 2017. Front Microbiol. 2017. PMID: 28373867 Free PMC article. Review. - Simple topology: FtsK-directed recombination at the dif site.
Grainge I. Grainge I. Biochem Soc Trans. 2013 Apr;41(2):595-600. doi: 10.1042/BST20120299. Biochem Soc Trans. 2013. PMID: 23514160 Review.
Cited by
- Replication termination and chromosome dimer resolution in the archaeon Sulfolobus solfataricus.
Duggin IG, Dubarry N, Bell SD. Duggin IG, et al. EMBO J. 2011 Jan 5;30(1):145-53. doi: 10.1038/emboj.2010.301. Epub 2010 Nov 26. EMBO J. 2011. PMID: 21113132 Free PMC article. - DNA Segregation in Enterobacteria.
Cornet F, Blanchais C, Dusfour-Castan R, Meunier A, Quebre V, Sekkouri Alaoui H, Boudsoq F, Campos M, Crozat E, Guynet C, Pasta F, Rousseau P, Ton Hoang B, Bouet JY. Cornet F, et al. EcoSal Plus. 2023 Dec 12;11(1):eesp00382020. doi: 10.1128/ecosalplus.esp-0038-2020. Epub 2023 May 9. EcoSal Plus. 2023. PMID: 37220081 Free PMC article. Review. - Conformational transitions during FtsK translocase activation of individual XerCD-dif recombination complexes.
Zawadzki P, May PF, Baker RA, Pinkney JN, Kapanidis AN, Sherratt DJ, Arciszewska LK. Zawadzki P, et al. Proc Natl Acad Sci U S A. 2013 Oct 22;110(43):17302-7. doi: 10.1073/pnas.1311065110. Epub 2013 Oct 7. Proc Natl Acad Sci U S A. 2013. PMID: 24101525 Free PMC article. - FtsK translocation permits discrimination between an endogenous and an imported Xer/dif recombination complex.
Fournes F, Crozat E, Pages C, Tardin C, Salomé L, Cornet F, Rousseau P. Fournes F, et al. Proc Natl Acad Sci U S A. 2016 Jul 12;113(28):7882-7. doi: 10.1073/pnas.1523178113. Epub 2016 Jun 17. Proc Natl Acad Sci U S A. 2016. PMID: 27317749 Free PMC article. - Activation of Xer-recombination at dif: structural basis of the FtsKγ-XerD interaction.
Keller AN, Xin Y, Boer S, Reinhardt J, Baker R, Arciszewska LK, Lewis PJ, Sherratt DJ, Löwe J, Grainge I. Keller AN, et al. Sci Rep. 2016 Oct 6;6:33357. doi: 10.1038/srep33357. Sci Rep. 2016. PMID: 27708355 Free PMC article.
References
- Lesterlin C, Barre F, Cornet F. Genetic recombination and the cell cycle: what we have learned from chromosome dimers. Mol. Microbiol. 2004;54:1151–1160. - PubMed
- Sherratt D. Bacterial chromosome dynamics. Science. 2003;301:780–785. - PubMed
- Bigot S, Sivanathan V, Possoz C, Barre F, Cornet F. FtsK, a literate chromosome segregation machine. Mol. Microbiol. 2007;64:1434–1441. - PubMed
- Grindley N, Whiteson K, Rice P. Mechanisms of site-specific recombination. Annu. Rev. Biochem. 2006;75:567–605. - PubMed