Probing the biology of Alzheimer's disease in mice - PubMed (original) (raw)
Review
Probing the biology of Alzheimer's disease in mice
Karen H Ashe et al. Neuron. 2010.
Abstract
Alzheimer's disease (AD), the most common cause of dementia among the elderly, may either represent the far end of a continuum that begins with age-related memory decline or a distinct pathobiological process. Although mice that faithfully model all aspects of AD do not yet exist, current mouse models have provided valuable insights into specific aspects of AD pathogenesis. We will argue that transgenic mice expressing amyloid precursor protein should be considered models of accelerated brain aging or asymptomatic AD, and the results of interventional studies in these mice should be considered in the context of primary prevention. Studies in mice have pointed to the roles of soluble beta-amyloid (Abeta) oligomers and soluble tau in disease pathogenesis and support a model in which soluble Abeta oligomers trigger synaptic dysfunction, but formation of abnormal tau species leads to neuron death and cognitive decline severe enough to warrant a dementia diagnosis.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
Figure 1
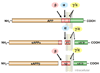
APP and its major proteolytic products. Cleavage of APP by α-, β-, γ-, and ε-secretases produces secreted amino-terminal fragments, carboxyl-terminal polypeptides, and Aβ. (Ma et al. (2007) Involvement of beta-site APP cleaving enzyme 1 (BACE1) in amyloid precursor protein-mediated enhancement of memory and activity-dependent synaptic plasticity. Proc Nat’l Acad Sci U S A. 104(19):8167–72. “Copyright (2007) National Academy of Sciences, U.S.A.”)
Figure 2
Regional hypometabolism in APP transgenic mice and people at risk for AD. MRI and [18F]-FDG-PET scans of (A) a control subject and (B) a carrier of a presenilin-1 mutation associated with familial autosomal dominant AD, 27 years before mean age of onset of dementia in her family. Bilateral hypometabolism of parietal cortex and medial temporal lobe is evident on PET in the absence of atrophy on MRI. C) Regional cerebral glucose utilization, monitored with [14C]2-deoxyglucose, in the cortex and hippocampus of a neophobic transgenic FVB/N mouse expressing human APP with a disease-linked mutation. Compared to its non-transgenic littermate, the transgenic mouse shows decreases in glucose utilization in cerebral cortex and hippocampus. Pseudocolored images of autoradiograms of coronal sections through cerebral cortex, hippocampus, and anterior brainstem; warmer colors represent greater glucose uptake. D) Magnified view of (C) showing hippocampus and overlying cerebral cortex. (A,B) reprinted by permission of the Society of Nuclear Medicine from: Mosconi L, Sorbi S, de Leon MJ, et al. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer's disease. J Nucl Med. 2006; 47(11): 1778–86. Figure 2. (C,D) reprinted from Neuron 15(5), Hsiao KK, Borchelt DR, Olson K, Johannsdottir R, Kitt C, Yunis W, Xu S, Eckman C, Younkin S, Price D, et al., Age-related CNS disorder and early death in transgenic FVB/N mice overexpressing Alzheimer amyloid precursor proteins, Pages 1203–18, Copyright (1995), with permission from Elsevier.
Figure 3
APP transgenic mice model asymptomatic Alzheimer’s disease. APP transgenic mice exhibit functional abnormalities similar to those observed in people at risk for Alzheimer’s disease, but do not display the loss of neurons seen in people clinically diagnosed with Mild Cognitive Impairment or Alzheimer’s disease.
Figure 4
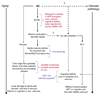
Ab initiates a disease process that might progress to a stage of cognitive impairment, but tau mediates cognitive dysfunction. In this hypothetical scheme, Aβ*56 (see Section 4.1) and perhaps other Aβ oligomers, cause synaptic dysfunction that manifests as subtle cognitive deficits in APP transgenic mice and in people in the asymptomatic phase of AD. Toxic Aβ oligomers surrounding plaques locally damage neurons, but their effects on cognition are unknown (see Section 4.2). Abnormal tau processing triggered by pathological forms of Aβ in AD, and by mutations in tau transgenic mice, cause loss of synapses and neurons and cognitive deficits severe enough to warrant a diagnosis of Mild Cognitive Impairment (MCI) or AD. The effects of NFTs, rather than soluble pathological forms of tau, are unknown (see Section 4.4). It is also not known whether the same effector molecules/mechanisms responsible for Aβ-induced synaptic and cognitive dysfunction trigger tau abnormalities or whether a separate pathway is involved.
Similar articles
- Soluble Conformers of Aβ and Tau Alter Selective Proteins Governing Axonal Transport.
Sherman MA, LaCroix M, Amar F, Larson ME, Forster C, Aguzzi A, Bennett DA, Ramsden M, Lesné SE. Sherman MA, et al. J Neurosci. 2016 Sep 14;36(37):9647-58. doi: 10.1523/JNEUROSCI.1899-16.2016. J Neurosci. 2016. PMID: 27629715 Free PMC article. - Lipidomic analysis of brain tissues and plasma in a mouse model expressing mutated human amyloid precursor protein/tau for Alzheimer's disease.
Tajima Y, Ishikawa M, Maekawa K, Murayama M, Senoo Y, Nishimaki-Mogami T, Nakanishi H, Ikeda K, Arita M, Taguchi R, Okuno A, Mikawa R, Niida S, Takikawa O, Saito Y. Tajima Y, et al. Lipids Health Dis. 2013 May 9;12:68. doi: 10.1186/1476-511X-12-68. Lipids Health Dis. 2013. PMID: 23659495 Free PMC article. - Molecular and functional signatures in a novel Alzheimer's disease mouse model assessed by quantitative proteomics.
Kim DK, Park J, Han D, Yang J, Kim A, Woo J, Kim Y, Mook-Jung I. Kim DK, et al. Mol Neurodegener. 2018 Jan 16;13(1):2. doi: 10.1186/s13024-017-0234-4. Mol Neurodegener. 2018. PMID: 29338754 Free PMC article. - Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?
Merino JJ, Muñetón-Gómez V, Alvárez MI, Toledano-Díaz A. Merino JJ, et al. Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review. - Modeling Alzheimer's disease in transgenic mice: effect of age and of presenilin1 on amyloid biochemistry and pathology in APP/London mice.
Dewachter I, van Dorpe J, Spittaels K, Tesseur I, Van Den Haute C, Moechars D, Van Leuven F. Dewachter I, et al. Exp Gerontol. 2000 Sep;35(6-7):831-41. doi: 10.1016/s0531-5565(00)00149-2. Exp Gerontol. 2000. PMID: 11053674 Review.
Cited by
- Near-infrared fluorescence molecular imaging of amyloid beta species and monitoring therapy in animal models of Alzheimer's disease.
Zhang X, Tian Y, Zhang C, Tian X, Ross AW, Moir RD, Sun H, Tanzi RE, Moore A, Ran C. Zhang X, et al. Proc Natl Acad Sci U S A. 2015 Aug 4;112(31):9734-9. doi: 10.1073/pnas.1505420112. Epub 2015 Jul 21. Proc Natl Acad Sci U S A. 2015. PMID: 26199414 Free PMC article. - Stem cell therapy for Alzheimer's disease and related disorders: current status and future perspectives.
Tong LM, Fong H, Huang Y. Tong LM, et al. Exp Mol Med. 2015 Mar 13;47(3):e151. doi: 10.1038/emm.2014.124. Exp Mol Med. 2015. PMID: 25766620 Free PMC article. Review. - Linking insulin with Alzheimer's disease: emergence as type III diabetes.
Ahmed S, Mahmood Z, Zahid S. Ahmed S, et al. Neurol Sci. 2015 Oct;36(10):1763-9. doi: 10.1007/s10072-015-2352-5. Epub 2015 Aug 7. Neurol Sci. 2015. PMID: 26248483 Review. - Magnetic Resonance Imaging in Animal Models of Alzheimer's Disease Amyloidosis.
Ni R. Ni R. Int J Mol Sci. 2021 Nov 25;22(23):12768. doi: 10.3390/ijms222312768. Int J Mol Sci. 2021. PMID: 34884573 Free PMC article. Review. - Identification and drug-induced reversion of molecular signatures of Alzheimer's disease onset and progression in AppNL-G-F, AppNL-F, and 3xTg-AD mouse models.
Pauls E, Bayod S, Mateo L, Alcalde V, Juan-Blanco T, Sánchez-Soto M, Saido TC, Saito T, Berrenguer-Llergo A, Attolini CS, Gay M, de Oliveira E, Duran-Frigola M, Aloy P. Pauls E, et al. Genome Med. 2021 Oct 26;13(1):168. doi: 10.1186/s13073-021-00983-y. Genome Med. 2021. PMID: 34702310 Free PMC article.
References
- Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, Davies P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem. 2003;86:582–590. - PubMed
- Banks WA, Farr SA, Butt W, Kumar VB, Franko MW, Morley JE. Delivery across the blood-brain barrier of antisense directed against amyloid beta: reversal of learning and memory deficits in mice overexpressing amyloid precursor protein. J Pharmacol Exp Ther. 2001;297:1113–1121. - PubMed
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG026252/AG/NIA NIH HHS/United States
- R01 MH065465/MH/NIMH NIH HHS/United States
- R01 MH065465-05/MH/NIMH NIH HHS/United States
- P01 AG015453-06/AG/NIA NIH HHS/United States
- R01 NS033249-15A1/NS/NINDS NIH HHS/United States
- R01 AG026252-05/AG/NIA NIH HHS/United States
- P01 AG015453/AG/NIA NIH HHS/United States
- R01 NS033249/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases