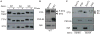TARP phosphorylation regulates synaptic AMPA receptors through lipid bilayers - PubMed (original) (raw)
Comparative Study
TARP phosphorylation regulates synaptic AMPA receptors through lipid bilayers
Akio Sumioka et al. Neuron. 2010.
Abstract
Neurons use neurotransmitters to communicate across synapses, constructing neural circuits in the brain. AMPA-type glutamate receptors are the predominant excitatory neurotransmitter receptors mediating fast synaptic transmission. AMPA receptors localize at synapses by forming protein complexes with transmembrane AMPA receptor regulatory proteins (TARPs) and PSD-95-like membrane-associated guanylate kinases. Among the three classes of ionotropic glutamate receptors (AMPA, NMDA, and kainate type), AMPA receptor activity is most regulatable by neuronal activity to adjust synaptic strength. Here, we mutated the prototypical TARP, stargazin, and found that TARP phosphorylation regulates synaptic AMPA receptor activity in vivo. We also found that stargazin interacts with negatively charged lipid bilayers in a phosphorylation-dependent manner and that the lipid interaction inhibited stargazin binding to PSD-95. Cationic lipids dissociated stargazin from lipid bilayers and enhanced synaptic AMPA receptor activity in a stargazin phosphorylation-dependent manner. Thus, TARP phosphorylation plays a critical role in regulating AMPA receptor-mediated synaptic transmission via a lipid bilayer interaction.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
Figure 1. Stargazin phosphorylation regulates synaptic localization of stargazin
All nine phosphorylated serine residues of stargazin (STG) were mutated to either aspartate (phospho-mimic; stargazinSD) or alanine (non-phospho mimic; stargazinSA) in knockin mice. (A) Lambda phosphatase treatment (PPase) lowered the molecular weight of stargazin from wild-type mice (WT), but not from StargazinSD (SD) or StargazinSA mice (SA). Western blots performed with three different anti-stargazin antibodies showed similar patterns. Western blots of fractionated brains from WT (B), and StargazinSD/StargazinSA hemizygous mice (C) showed that stargazin in synaptic fraction (PSD) migrated as higher molecular weight than that in non-synaptic fraction (Syn/Tx) (B). (C) StargazinSD was highly enriched in the postsynaptic density (PSD) fraction, whereas stargazinSA was distributed evenly between the PSD and Triton-X-100-soluble synaptosome (Syn/Tx) fractions. Sph, synaptophysin. Geno, genotype.
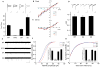
Figure 2. Stargazin phosphorylation modulates AMPA receptor activity in cerebellar mossy fiber/granule cell synapses in vivo
(A) EPSCs elicited by mossy fiber stimulation were recorded in cerebellar granule cells from wild-type (WT), stargazer (STG), and stargazin knockin mice (SA and SD). The AMPA receptor component of EPSCs (IAMPA) was measured as the peak amplitude at a holding potential of −70 mV and the NMDA receptor component (INMDA) was measured at a holding potential of +40 mV and at 50 ms latency. The ratio of IAMPA to INMDA was increased by ~75% in stargazinSD mice compared with wild-type mice (P < 0.01; n = 6 for wild-type mice and n = 7 for stargazinSD mice), and reduced by ~38% in stargazinSA mice compared with wild-type mice (P < 0.01; n = 6 for wild-type mice and n = 6 for stargazinSA mice). The IAMPA was invisible in stargazer mice (n = 6). Sample traces of EPSCs are shown in A at a holding potential of −70 mV (bottom) or +40 mV (top). Scale bar, 20 ms and 40 pA (WT), 10 pA (STG), 20 pA (SA), and 50 pA (SD). (B, C) I–V relationships of MF–EPSCs from each genotype, measured at the peak of (B), and 50 ms (C) after, the stimulus. The EPSC amplitudes were normalized to the mean value at +50 mV in each genotype (n = 6–7). (D) Paired-pulse facilitation (PPF) values measured at 40 ms intervals did not differ among the genotypes (n = 6–7). (E, F, and G) mEPSCs recorded from cerebellar granule cells in acute cerebellar slices at a holding potential of −70 mV in the presence of 1 μM TTX. Sample traces are shown in (E). (F) Cumulative distribution of mEPSC amplitudes and average mEPSC amplitude (small inset). The mEPSC amplitude was significantly larger in stargazinSD mice compared with wild-type mice (P < 0.01, Kolmogorov–Smirnov test for cumulative distribution; P < 0.01, one-way ANOVA for average, n = 10 for wild-type mice and n = 11 for stargazinSD mice), whereas it was significantly smaller in stargazinSA mice compared with wild-type mice (P < 0.01, Kolmogorov–Smirnov test for cumulative distribution; P < 0.05, one-way ANOVA for average, n = 10 for wild-type mice and n = 9 for stargazinSA mice). However, the time intervals between events (G) were not significantly different among the genotypes. Error bars in all graphs represent the SEM.
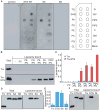
Figure 3. Stargazin binds negatively-charged lipids in a phosphorylation-dependent manner
(A) The cytoplasmic domain of stargazin (STG) binds directly to phosphatidic acid (PA) and phosphoinositides (PIP, PIP2, PIP3). GST-fused stargazin cytoplasmic domains were overlaid on a membrane spotted with various lipids and bound stargazin was detected with anti-GST antibody. TG, triglyceride; DAG, diacylglycerol; PA, phosphatidic acid; PS, phosphatidylserine; PE, phosphatidylethanolamine; PC, phosphatidylcholine; PG, phosphatidylglycerol; CL, cardiolipin; PI, phosphatidylinositol; PIP, phosphatidylinositol 4-phosphate; PIP2, phosphatidylinositol 4,5-biphosphate; PIP3, phosphatidylinositol 3,4,5-triphosphate; C, cholesterol; SM, sphingomyelin; and ST, 3-sulfogalactosylceramide. (B) The cytoplasmic domain of stargazin recognizes negatively-charged liposomes. Thioredoxin (Trx)-fused stargazin cytoplasmic domains were mixed with liposomes containing phosphatidyl choline (PC) and various lipids (9:1). Subsequently, liposome-bound and unbound proteins were separated by sucrose gradient centrifugation and examined by western blotting. (C) Quantitation of liposome-bound proteins normalized against PIP2/PC-binding stargazin. (D) The cytoplasmic domain of stargazin interacts with PC/PA liposomes in a phosphorylation-dependent manner. PC/PA liposomes interact with thioredoxin-fused cytoplasmic domains of stargazin and stargazinSA, but not stargazinSD. (E) Quantitation of liposome-bound proteins normalized against stargazin. (F) The cytoplasmic domain of stargazin interacted with PC/PA liposomes via its positively charged residues (arginines). Eight of the arginine residues located around the stargazin phosphorylation sites and all arginine residues were replaced with seven leucines and one glycine residue (RL). PC/PA liposomes interacted with the thioredoxin-fused cytoplasmic domain of stargazin (STG), but not stargazinRL, which indicates that the cytoplasmic domain of stargazin interacted with lipid bilayers via an electrostatic interaction. Error bars in (C) (n = 3) and (E) (n = 5) show means ± SEM.
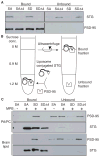
Figure 4. Lipid bilayers inhibit binding of stargazin to PSD-95
(A) In the absence of lipids, the four C-terminal amino acids of stargazinSD (SD) and stargazinSA (SA) bind PSD-95. The PSD-95 PDZ domain-bound and unbound stargazin (STG) cytoplasmic domains were separated with glutathione beads. Both stargazinSD and stargazinSA bound to the PDZ domain, whereas mutants lacking the last four amino acids (Δ4) did not bind. (B) This diagram shows the experimental scheme to examine the effects of lipid bilayers on the stargazin binding to PSD-95. Liposomes conjugated to the stargazin cytoplasmic domain were incubated with the PSD-95 PDZ domains. Stargazin-bound and unbound PSD-95 were separated by sucrose gradient centrifugation. (C) Lipid bilayers inhibit stargazin interaction with PSD-95. Stargazin did not conjugate with liposomes lacking MPB-PE (MPB). The PSD-95 PDZ domains bound liposomes conjugated with stargazinSD but not stargazinSA or stargazinSDΔ4. Liposomes constituted with PC/PA or brain lipids showed similar results.
Figure 5. PSD-95 binding requires stargazin dissociation from lipid bilayers
The cationic lipid lipofectamine increased the interaction between stargazinSA (SA) and PSD-95. (A) Shown is the experimental scheme for examining the effects of cationic lipids on stargazin (STG) binding to PSD-95. Lipofectamine (approx. 100 μM) was added to a stargazin-conjugated liposome and PSD-95 mixture. Stargazin-bound and unbound PSD-95 were separated by sucrose gradient centrifugation. (B) PSD-95 did not bind stargazinSA in the phosphatidylcholine/phosphatidic acid liposomes. Upon addition of lipofectamine, PSD-95 bound the stargazinSA from liposomes but not the stargazinSAΔ4. The interaction between the stargazinSD-containing liposomes and PSD-95 was unaltered by lipofectamine. Notably, the weak signal observed for the stargazinSA Δ4 was also observed in liposomes conjugated with cysteine alone, which suggests that this weak signal is non-specific (Figure S5). (C) The cytoplasmic domain of stargazin localized at the plasma membrane in a phosphorylation-dependent manner. The cytoplasmic domains of stargazin and mutants were tagged with a myristoylation motif at the N terminus, to mimic the localization of the cytoplasmic domain near a transmembrane domain in stargazin, and with GFP at the C terminus, to monitor its distribution (myrSTG), and expressed in CHO cells together with mCherry-tagged R-Pre, which interacts with negatively charged membranes (C). The myristoylated stargazinSA mutant (myrSA) colocalized with mCherry-R-pre, whereas GFP alone, myrSD, and myrSTG did not. The relative distribution of stargazin was analyzed relative to that of mCherry-R-Pre. (D) The cationic lipid sphingosine translocated myrSA from the plasma membrane to the cytoplasm, similarly to R-pre. Addition of the cationic lipid sphingosine (100 μM for 5–20 min) induced the relocalization of myrSA from the plasma membrane to the cytoplasm. The relative distribution of stargazin and R-pre was shown after adjustment of total amount of signal from single cell as 1 because total amount of proteins were not changed before and after addition of cationic lipid. All data are shown as means ± SEM (n = 10 cells).
Figure 6. Cationic lipids enhance synaptic activity of AMPA receptors in a stargazin phosphorylation-dependent manner
(A) The cationic lipid sphingosine-NBD inserts into neuronal membranes. Sphingosine-NBD (2.5 μM) or vehicle (ethanol) was added to cerebellar granule cells and analyzed by confocal microscopy. Top panels, DIC; Bottom panels, NBD channel. (B–D) AMPA receptor-mediated miniature EPSCs (mEPSC) were recorded from cerebellar granule cells from stargazinSA (SA), stargazinSD (SD) and wild-type mice (WT) before and after addition of cationic lipids, sphingosine (2.5 μM) or squalamine (2.5 μM). Shown are the representative traces (B), mean amplitude (C), and weighted tau (D) of AMPA receptor-mediated mEPSC from each genotype before and after sphingosine addition. In StargazinSA mice, the amplitude of mEPSC increased upon addition of sphingosine, but no changes in decay kinetics were observed. No similar increase in amplitude was observed for WT and StargazinSD mice (n = 164–188 and 1626–1869 events from 13–15 cells for each genotype before and after sphingosine treatment, respectively). *** P<0.005. (E) Mean amplitude of AMPA receptor-mediated mEPSC from each genotype before and after squalamine addition (2.5 μM) (n = 48 (before squalamine) and n = 169 (after squalamine) events from six stargazinSA cells; n = 49 (before squalamine) and n = 160 (after squalamine) events from seven stargazinSD cells.). * P < 0.01. (F) AMPA-evoked currents did not change upon addition of sphingosine (n = 13–15 cells for each genotype before and after sphingosine treatment, respectively). Data are shown as means ± SEM.
Figure 7. Cationic lipids enhance translocation of stargazin to synapses in a stargazin phosphorylation-dependent manner
(A and B) Treatment with a cationic lipid increased the synaptic expression of stargazinSA, but not of stargazinSD, without changes in the synaptic expression of PSD-95. Cerebellar granule cells from stargazinSA and stargazinSD mice were treated with and without sphingosine (10 μM for 5 min), which was followed by fractionation of soluble Triton X-100 (Syn/Tx) and insoluble PSD-enriched (PSD) fractions. Stargazin (STG) translocated into the PSD fraction after addition of sphingosine in neurons from stargazinSA, but not stargazinSD, mice, without changes in PSD-95 and synaptophysin (Sph). Protein amounts were quantitated using ImageJ. Data are shown as means ± SEM (n = 6). (C) Stargazin immunoprecipitated PSD-95 from cerebellar granule cells treated with a crosslinker (CL), which indicated that stargazin did not interact artificially with PSD-95 during incubation (in test tubes) under this experimental condition. (D) Cationic lipid treatment (10 μM sphingosine 5 min with 2 μM TTX) increased the interaction between PSD-95 and StargazinSA, but not StargazinSD, without changes in the total levels of protein expression. (E) Quantitative analyses showed that total protein expression was no significantly different after the treatment with cationic lipids, whereas the level of PSD-95 immunoprecipitated with the anti-stargazin antibody was significantly increased. Data are shown as means ± SEM (n = 3). *; P < 0.01. (F) A model for the TARP phosphorylation-mediated regulation of synaptic AMPA receptors via lipid bilayers. The interaction of negatively charged lipid bilayers with stargazin inhibits the binding of stargazin to PSD-95. Dissociation of lipids from phosphorylated stargazin leads to its binding to PSD-95 at synapses.
Similar articles
- Stargazin and other transmembrane AMPA receptor regulating proteins interact with synaptic scaffolding protein MAGI-2 in brain.
Deng F, Price MG, Davis CF, Mori M, Burgess DL. Deng F, et al. J Neurosci. 2006 Jul 26;26(30):7875-84. doi: 10.1523/JNEUROSCI.1851-06.2006. J Neurosci. 2006. PMID: 16870733 Free PMC article. - Phase Separation-Mediated TARP/MAGUK Complex Condensation and AMPA Receptor Synaptic Transmission.
Zeng M, Díaz-Alonso J, Ye F, Chen X, Xu J, Ji Z, Nicoll RA, Zhang M. Zeng M, et al. Neuron. 2019 Nov 6;104(3):529-543.e6. doi: 10.1016/j.neuron.2019.08.001. Epub 2019 Sep 3. Neuron. 2019. PMID: 31492534 Free PMC article. - Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs.
Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS. Tomita S, et al. Neuron. 2005 Jan 20;45(2):269-77. doi: 10.1016/j.neuron.2005.01.009. Neuron. 2005. PMID: 15664178 - Stargazin interacts functionally with the AMPA receptor glutamate-binding module.
Tomita S, Shenoy A, Fukata Y, Nicoll RA, Bredt DS. Tomita S, et al. Neuropharmacology. 2007 Jan;52(1):87-91. doi: 10.1016/j.neuropharm.2006.07.012. Epub 2006 Aug 21. Neuropharmacology. 2007. PMID: 16919685 Review. - Auxiliary subunits provide new insights into regulation of AMPA receptor trafficking.
Sumioka A. Sumioka A. J Biochem. 2013 Apr;153(4):331-7. doi: 10.1093/jb/mvt015. Epub 2013 Feb 20. J Biochem. 2013. PMID: 23426437 Review.
Cited by
- Homeostatic control of synaptic transmission by distinct glutamate receptors.
Yan D, Yamasaki M, Straub C, Watanabe M, Tomita S. Yan D, et al. Neuron. 2013 May 22;78(4):687-99. doi: 10.1016/j.neuron.2013.02.031. Neuron. 2013. PMID: 23719165 Free PMC article. - Chemical shift assignments of the N-terminal domain of PSD95 (PSD95-NT).
Zhang Y, Hell JW, Ames JB. Zhang Y, et al. Biomol NMR Assign. 2021 Oct;15(2):347-350. doi: 10.1007/s12104-021-10028-5. Epub 2021 Apr 30. Biomol NMR Assign. 2021. PMID: 33929702 Free PMC article. - Palmitoylation-dependent regulation of glutamate receptors and their PDZ domain-containing partners.
Thomas GM, Huganir RL. Thomas GM, et al. Biochem Soc Trans. 2013 Feb 1;41(1):72-8. doi: 10.1042/BST20120223. Biochem Soc Trans. 2013. PMID: 23356261 Free PMC article. Review. - Targeting _N_-Methyl-d-Aspartate Receptors in Neurodegenerative Diseases.
Carles A, Freyssin A, Perin-Dureau F, Rubinstenn G, Maurice T. Carles A, et al. Int J Mol Sci. 2024 Mar 27;25(7):3733. doi: 10.3390/ijms25073733. Int J Mol Sci. 2024. PMID: 38612544 Free PMC article. Review. - Postsynaptic localization and regulation of AMPA receptors and Cav1.2 by β2 adrenergic receptor/PKA and Ca2+/CaMKII signaling.
Patriarchi T, Buonarati OR, Hell JW. Patriarchi T, et al. EMBO J. 2018 Oct 15;37(20):e99771. doi: 10.15252/embj.201899771. Epub 2018 Sep 24. EMBO J. 2018. PMID: 30249603 Free PMC article. Review.
References
- Bats C, Groc L, Choquet D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron. 2007;53:719–734. - PubMed
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. - PubMed
- Cho CH, St-Gelais F, Zhang W, Tomita S, Howe JR. Two Families of TARP Isoforms that Have Distinct Effects on the Kinetic Properties of AMPA Receptors and Synaptic Currents. Neuron. 2007;55:890–904. - PubMed
- Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–942. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH077939-02/MH/NIMH NIH HHS/United States
- R01 MH077939-01A2/MH/NIMH NIH HHS/United States
- MH077939/MH/NIMH NIH HHS/United States
- R01 MH077939/MH/NIMH NIH HHS/United States
- R56 MH077939/MH/NIMH NIH HHS/United States
- P30 DA018343/DA/NIDA NIH HHS/United States
- P30DA018343/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous