Development of calcium-permeable AMPA receptors and their correlation with NMDA receptors in fast-spiking interneurons of rat prefrontal cortex - PubMed (original) (raw)
Development of calcium-permeable AMPA receptors and their correlation with NMDA receptors in fast-spiking interneurons of rat prefrontal cortex
Huai-Xing Wang et al. J Physiol. 2010.
Abstract
Abnormal influx of Ca(2+) is thought to contribute to the neuronal injury associated with a number of brain disorders, and Ca(2+)-permeable AMPA receptors (CP-AMPARs) play a critical role in the pathological process. Despite the apparent vulnerability of fast-spiking (FS) interneurons in neurological disorders, little is known about the CP-AMPARs expressed by functionally identified FS interneurons in the developing prefrontal cortex (PFC). We investigated the development of inwardly rectifying AMPA receptor-mediated currents and their correlation with NMDA receptor-mediated currents in FS interneurons in the rat PFC. We found that 78% of the FS interneurons expressed a low rectification index, presumably Ca(2+)-permeable AMPARs, with only 22% exhibiting AMPARs with a high rectification index, probably Ca(2+) impermeable (CI). FS interneurons with CP-AMPARs exhibited properties distinct from those expressing CI-AMPARs, although both displayed similar morphologies, passive membrane properties and AMPA currents at resting membrane potentials. The AMPA receptors also exhibited dramatic changes during cortical development with significantly more FS interneurons with CP-AMPARs and a clearly decreased rectification index during adolescence. In addition, FS interneurons with CP-AMPARs exhibited few or no NMDA currents, distinct frequency-dependent synaptic facilitation, and protracted maturation in short-term plasticity. These data suggest that CP-AMPARs in FS interneurons may play a critical role in neuronal integration and that their characteristic properties may make these cells particularly vulnerable to disruptive influences in the PFC, thus contributing to the onset of many psychiatric disorders.
Figures
Figure 1. Two subgroups of FS interneurons in the rat medial PFC
A−C, a sample of FS interneurons exhibiting basket-like morphology (A), high-frequency firing (B), and large CI-AMPARs with high RIs (C, RI = 1.08). The I−V curve of AMPA-mediated currents in C was derived from the same cell used in B. D−F, a sample of FS interneurons with CP-AMPARs. Although the neurons showed similar basket-like morphology (D) and fired high-frequency action potentials (E), the AMPAR-mediated currents exhibited low RIs (arrow in F, RI = 0.17). Inset, the RI value in the CI interneurons (_n_= 19) was significantly higher (P < 0.0001) than that in the CP cells (_n_= 68). Scale bar in D represents 10 μm for both A and D. G, sample traces of evoked EPSCs at different holding potentials from −80 to +80 mV with 20 mV steps in FS interneurons containing CI- and CP-AMPARs, respectively. H, normalized I_–_V relationship for the EPSCs recorded as shown in G. The EPSCs were normalized to −80 mV levels and the data were derived from 4–5 neurons. Dashed lines: a linear fit function was applied to the EPSC amplitudes in the hyperpolarized voltage range, and the deviation of the EPSC amplitudes in the depolarized voltage range from such a fit was only obvious in the FS interneurons with CP-AMPARs (arrow). Both I_–_V relations in CI and CP interneurons exhibited similar reversal potentials without statistical difference (_P_= 0.121), but a weak correlation exists between RI values and reversal potential (_P_= 0.073).
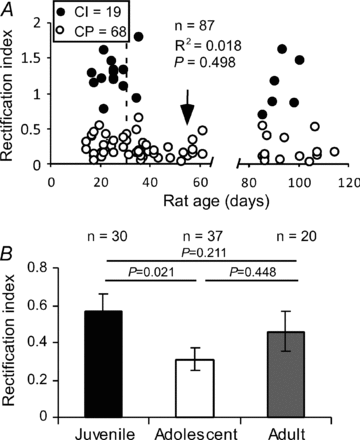
Figure 2. Developmental changes of FS interneurons containing CI- and CP-AMPARs
A, the changes of CI- and CP-AMPARs in the FS interneurons do not correlate with animal ages overall (_R_2= 0.018, _P_= 0.498), but a dramatic decrease in RI values was observed during the adolescent period. Most of the cells (89.2%) in this age group exhibited low RIs (arrow). B, FS interneurons exhibited a significant decrease in RI during the adolescent period (_P_= 0.021); the RI values were recovered in adults.
Figure 3. Correlation of CI- and CP-AMPARs with developmental changes of NMDA receptors in the FS interneurons
A–C, sample FS interneurons exhibiting large NMDA receptor-mediated currents in CI interneurons, but with small (B) or no (C) NMDA currents in the CP interneurons. D and E, the amplitudes of the NMDA receptor-mediated currents correlated well with the RI (_R_2= 0.464, P < 0.0001). The FS interneurons with CI-AMPARs expressed significantly more NMDA receptors compared with FS cells with CP-AMPARs, a bimodal distribution. In addition, there was a significant difference between CI and CP in the NMDA current amplitude (*P < 0.0001) and NMDA/AMPA ratio (*P < 0.0001). F and G, the amplitudes of NMDA EPSCs in FS interneurons were significantly decreased in adolescent and adult rats. Among these CP cells, NMDA EPSCs decreased progressively, and more and more CP interneurons expressed no NMDA currents in the adult animals.
Figure 4. Distinct NMDA/AMPA ratios in FS interneurons expressing CI- and CP-AMPARs
A, AMPAR-mediated EPSCs were recorded at −60, 0 and +60 mV, respectively, in the presence of picrotoxin and
d
-APV. B, mEPSCs were recorded at −60 mV in Mg2+-free external solution with bath perfusion of TTX and picrotoxin. Upper panel, example traces and expanded areas showing the mEPSCs in both CI and CP cells. Lower panel, averaged mEPSCs and measurements of both AMPA and NMDA currents. C and D, the mEPSCs in the FS interneurons with CI-AMPARs (_n_= 6) displayed significantly slower decay than those in CP cells (P < 0.05). The amplitudes in AMPA mEPSCs between CI and CP cells were similar (_P_= 0.948), but the amplitudes of NMDA mEPSCs were significantly different (_P_= 0.036). The FS interneurons expressing CI AMPA expressed significantly higher NMDA/AMPA ratios compared with those with CP AMPA (P < 0.005), and the RI values were significantly correlated with NMDA/AMPA ratios (_R_2= 0.847, _P_= 0.0001). E and F, in another set of experiments, the NMDA mEPSCs were recorded at +60 mV in the presence of picrotoxin, NBQX and TTX. Example traces and expanded areas in E showing the FS interneurons with and without NMDA mEPSCs. Among the 15 FS interneurons recorded, 9 cells exhibited no NMDA mEPSCs whereas the remaining 6 cells exhibited small NMDA currents (F).
Figure 5. Correlation between PPR and RI of AMPARs, as well as NMDA receptors, in the FS interneurons
A and B, the AMPAR-mediated EPSCs in FS interneurons exhibited both PPF and PPD with a majority of the synapses exhibiting facilitation and about one-third showing depression (*P < 0.0001 in B). C and D, facilitating and depressing FS interneurons seemed to be age-independent overall (_P_= 0.342) despite ∼20% increase of facilitating FS interneurons during the adolescent period. However, the PPR was significantly higher in adults (_P_= 0.013), and overall PPRs were correlated with postnatal ages (_n_= 54, _R_2= 0.131, _P_= 0.007). E, the PPRs were correlated with CI- and CP-AMPARs (_P_= 0.019) although both PPF and PPD synapses were seen in FS interneurons with CI- and CP-AMPARs. F, three samples of AMPAR- and NMDAR-mediated currents in FS interneurons exhibited PPF or PPD. Right panel, an FS interneuron with CP-AMPAR was first recorded in the presence of picrotoxin and
d
-APV and then after a 20 min washout, the NMDA EPSC was recorded at +60 mV in the presence of picrotoxin and NBQX. G, summary graph showing that PPF and PPD were closely correlated with the distribution of NMDA receptors in these interneurons, with PPF synapses containing fewer NMDA receptors than those in PPD synapses (χ2= 4.59, _P_= 0.032).
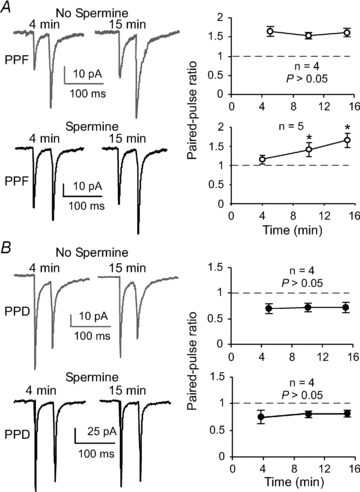
Figure 6. Spermine selectively modifies AMPA EPSCs in the facilitating FS interneurons in a time-dependent manner
A, the PPRs in synapses expressing PPF were relatively stable without significant changes when spermine was not included in the intracellular solution (_n_= 4, P > 0.05). Facilitating synapses in FS interneurons were, however, sensitive to spermine loaded in the recording pipette solution. The effects appeared to be time dependent. The PPRs in the FS cells expressing PPF were gradually and significantly increased (_n_= 5, _P_= 0.020; *P < 0.05). _B_, in contrast, synapses expressing PPD were not affected by spermine application, and the synapses remained depressed under both conditions (_n_= 4, _P_ > 0.05).
Figure 7. Frequency-dependent paired-pulse plasticity in facilitating and depressing FS interneurons
A, an example of EPSCs in a facilitating FS interneuron showing the frequency-dependent change of the second EPSC amplitudes relative to the first EPSCs. The synapse exhibited facilitation at frequencies higher than 10 Hz (100 ms interval) but expressed depression at lower frequencies such as 2 Hz and completely recovered at 1 Hz (1000 ms interval). B, overlap EPSC traces showing the changes of the second EPSCs relative to the first EPSCs. C, summary graph showing frequency-dependent change of PPR in the facilitating synapses (_n_= 5, *P < 0.05 between 20 Hz and 10, 5, 2 and 1 Hz, respectively). _D_, example of a depressing FS interneuron showing the EPSCs recorded in response to paired-pulse stimulation at different frequencies. In contrast to the facilitating FS interneurons, the synapse continued to be depressed, relatively independently of frequency change. _E_, overlap traces of paired-pulse EPSCs at different frequencies in a depressing FS interneuron. _F_, summary graph showing the frequency-independent change of PPR in 6 depressing FS interneurons (_n_= 6, _P_ > 0.05 between 20 Hz and 10, 5, 2 and 1 Hz, respectively).
Similar articles
- Cell type-specific development of NMDA receptors in the interneurons of rat prefrontal cortex.
Wang HX, Gao WJ. Wang HX, et al. Neuropsychopharmacology. 2009 Jul;34(8):2028-40. doi: 10.1038/npp.2009.20. Epub 2009 Feb 25. Neuropsychopharmacology. 2009. PMID: 19242405 Free PMC article. - Postsynaptic glutamate receptors and integrative properties of fast-spiking interneurons in the rat neocortex.
Angulo MC, Rossier J, Audinat E. Angulo MC, et al. J Neurophysiol. 1999 Sep;82(3):1295-302. doi: 10.1152/jn.1999.82.3.1295. J Neurophysiol. 1999. PMID: 10482748 - Prolonged exposure to NMDAR antagonist induces cell-type specific changes of glutamatergic receptors in rat prefrontal cortex.
Wang HX, Gao WJ. Wang HX, et al. Neuropharmacology. 2012 Mar;62(4):1808-22. doi: 10.1016/j.neuropharm.2011.11.024. Epub 2011 Dec 9. Neuropharmacology. 2012. PMID: 22182778 Free PMC article. - GluA2-lacking, calcium-permeable AMPA receptors--inducers of plasticity?
Man HY. Man HY. Curr Opin Neurobiol. 2011 Apr;21(2):291-8. doi: 10.1016/j.conb.2011.01.001. Epub 2011 Feb 2. Curr Opin Neurobiol. 2011. PMID: 21295464 Free PMC article. Review. - The AMPAR subunit GluR2: still front and center-stage.
Tanaka H, Grooms SY, Bennett MV, Zukin RS. Tanaka H, et al. Brain Res. 2000 Dec 15;886(1-2):190-207. doi: 10.1016/s0006-8993(00)02951-6. Brain Res. 2000. PMID: 11119696 Review.
Cited by
- Dopamine, cognitive function, and gamma oscillations: role of D4 receptors.
Furth KE, Mastwal S, Wang KH, Buonanno A, Vullhorst D. Furth KE, et al. Front Cell Neurosci. 2013 Jul 2;7:102. doi: 10.3389/fncel.2013.00102. eCollection 2013. Front Cell Neurosci. 2013. PMID: 23847468 Free PMC article. - Gestational methylazoxymethanol exposure leads to NMDAR dysfunction in hippocampus during early development and lasting deficits in learning.
Snyder MA, Adelman AE, Gao WJ. Snyder MA, et al. Neuropsychopharmacology. 2013 Jan;38(2):328-40. doi: 10.1038/npp.2012.180. Epub 2012 Sep 12. Neuropsychopharmacology. 2013. PMID: 22968815 Free PMC article. - Emergence of Coordinated Activity in the Developing Entorhinal-Hippocampal Network.
Valeeva G, Janackova S, Nasretdinov A, Rychkova V, Makarov R, Holmes GL, Khazipov R, Lenck-Santini PP. Valeeva G, et al. Cereb Cortex. 2019 Feb 1;29(2):906-920. doi: 10.1093/cercor/bhy309. Cereb Cortex. 2019. PMID: 30535003 Free PMC article. - Developmental expression of N-methyl-D-aspartate (NMDA) receptor subunits in human white and gray matter: potential mechanism of increased vulnerability in the immature brain.
Jantzie LL, Talos DM, Jackson MC, Park HK, Graham DA, Lechpammer M, Folkerth RD, Volpe JJ, Jensen FE. Jantzie LL, et al. Cereb Cortex. 2015 Feb;25(2):482-95. doi: 10.1093/cercor/bht246. Epub 2013 Sep 17. Cereb Cortex. 2015. PMID: 24046081 Free PMC article. - PSD-95 deficiency disrupts PFC-associated function and behavior during neurodevelopment.
Coley AA, Gao WJ. Coley AA, et al. Sci Rep. 2019 Jul 1;9(1):9486. doi: 10.1038/s41598-019-45971-w. Sci Rep. 2019. PMID: 31263190 Free PMC article.
References
- Andersen TF, Vogensen SB, Jensen LS, Knapp KM, Stromgaard K. Design and synthesis of labeled analogs of PhTX-56, a potent and selective AMPA receptor antagonist. Bioorg Med Chem. 2005;13:5104–5112. - PubMed
- Angulo MC, Rossier J, Audinat E. Postsynaptic glutamate receptors and integrative properties of fast-spiking interneurons in the rat neocortex. J Neurophysiol. 1999a;82:1295–1302. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous