Modified intention to treat reporting in randomised controlled trials: systematic review - PubMed (original) (raw)
Modified intention to treat reporting in randomised controlled trials: systematic review
Iosief Abraha et al. BMJ. 2010.
Abstract
Objectives: To determine the incidence and characteristics of randomised controlled trials that report using the modified intention to treat approach, and how the approach is described.
Design: Systematic review.
Data sources: PubMed, Embase, Cochrane central register of controlled trials, ISI Web of Knowledge, Ovid, HighWire Press, Science-Direct, Ingenta, Medscape, BioMed Central, Springer, and Wiley, from inception to December 2006.
Main outcome measures: Incidence of trials in which use of modified intention to treat was reported, and how the approach was described (classified according to the type and number of deviations from the intention to treat approach).
Results: 475 randomised controlled trials reported use of a modified intention to treat analysis. Of these, 76 (16%) were published in five highly cited general medical journals. The incidence of all trials that reported use of modified intention to treat published in journals indexed in Medline increased from 0.006% in 1982-6 to 0.5% in 2002-6 (P<0.001 for linear trend). When the description of the modified intention to treat was examined in each trial, 192 (40%) reported one type of deviation from the intention to treat approach, 261 (55%) reported two or more types, and 22 (5%) did not describe any type. In 266 (56%) of the trials the deviation was related to the treatment received, in 196 (41%) to a post baseline assessment, in 118 (25%) to a baseline assessment, in 108 (23%) to a target condition, and in 23 (5%) to follow-up. Post-randomisation exclusions occurred in 380 (80%) trials. The results reported by 270 of the 352 (77%) superiority trials favoured the drug under investigation. All of the 123 trials using equivalence or non-inferiority methods to investigate interventions reported results that favoured their assumptions.
Conclusions: Randomised controlled trials that report using a modified intention to treat are increasingly being published in the medical literature. The descriptions of such an approach were ambiguous, and may cover any type of descriptions for exclusion, such as missing data and deviation from protocol. Explicit statements about post-randomisation exclusions should replace the ambiguous terminology of modified intention to treat.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi\_disclosure.pdf (available on request from the corresponding author) and declare that (1) none of the authors have support from any company for the submitted work; (2) none of the authors have relationships with any companies that might have an interest in the submitted work in the previous 3 years; (3) none of their spouses, partners, or children have financial relationships that may be relevant to the submitted work; and (4) none of the authors have no non-financial interests that may be relevant to the submitted work.
Figures
Fig 1 Study screening process
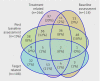
Fig 2 Number (percentage) of four most common types of deviations from intention to treat. Each type of deviation overlapped with at least one other type
Similar articles
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article. - Deviation from intention to treat analysis in randomised trials and treatment effect estimates: meta-epidemiological study.
Abraha I, Cherubini A, Cozzolino F, De Florio R, Luchetta ML, Rimland JM, Folletti I, Marchesi M, Germani A, Orso M, Eusebi P, Montedori A. Abraha I, et al. BMJ. 2015 May 27;350:h2445. doi: 10.1136/bmj.h2445. BMJ. 2015. PMID: 26016488 Free PMC article. - Modified versus standard intention-to-treat reporting: are there differences in methodological quality, sponsorship, and findings in randomized trials? A cross-sectional study.
Montedori A, Bonacini MI, Casazza G, Luchetta ML, Duca P, Cozzolino F, Abraha I. Montedori A, et al. Trials. 2011 Feb 28;12:58. doi: 10.1186/1745-6215-12-58. Trials. 2011. PMID: 21356072 Free PMC article. - A systematic review found that deviations from intention-to-treat are common in randomized trials and systematic reviews.
Abraha I, Cozzolino F, Orso M, Marchesi M, Germani A, Lombardo G, Eusebi P, De Florio R, Luchetta ML, Iorio A, Montedori A. Abraha I, et al. J Clin Epidemiol. 2017 Apr;84:37-46. doi: 10.1016/j.jclinepi.2016.11.012. Epub 2017 Jan 11. J Clin Epidemiol. 2017. PMID: 28088592 Review. - What is meant by intention to treat analysis? Survey of published randomised controlled trials.
Hollis S, Campbell F. Hollis S, et al. BMJ. 1999 Sep 11;319(7211):670-4. doi: 10.1136/bmj.319.7211.670. BMJ. 1999. PMID: 10480822 Free PMC article. Review.
Cited by
- The effect of the combination of whole body vibration and shoe with an unstable surface in chronic ankle instability treatment: a randomized clinical trial.
Shamseddini Sofla F, Hadadi M, Rezaei I, Azhdari N, Sobhani S. Shamseddini Sofla F, et al. BMC Sports Sci Med Rehabil. 2021 Mar 19;13(1):28. doi: 10.1186/s13102-021-00256-6. BMC Sports Sci Med Rehabil. 2021. PMID: 33741051 Free PMC article. - Traditional Chinese Medicine Compound (Tongxinluo) and Clinical Outcomes of Patients With Acute Myocardial Infarction: The CTS-AMI Randomized Clinical Trial.
Yang Y, Li X, Chen G, Xian Y, Zhang H, Wu Y, Yang Y, Wu J, Wang C, He S, Wang Z, Wang Y, Wang Z, Liu H, Wang X, Zhang M, Zhang J, Li J, An T, Guan H, Li L, Shang M, Yao C, Han Y, Zhang B, Gao R, Peterson ED; CTS-AMI Investigators. Yang Y, et al. JAMA. 2023 Oct 24;330(16):1534-1545. doi: 10.1001/jama.2023.19524. JAMA. 2023. PMID: 37874574 Free PMC article. Clinical Trial. - Covered metallic stents with an anti-migration design vs. uncovered stents for the palliation of malignant gastric outlet obstruction: a multicenter, randomized trial.
Lee H, Min BH, Lee JH, Shin CM, Kim Y, Chung H, Lee SH. Lee H, et al. Am J Gastroenterol. 2015 Oct;110(10):1440-9. doi: 10.1038/ajg.2015.286. Epub 2015 Sep 15. Am J Gastroenterol. 2015. PMID: 26372507 Free PMC article. Clinical Trial. - Exclusion of enrolled participants in randomised controlled trials: what to do with ineligible participants?
Rehman AM, Ferrand R, Allen E, Simms V, McHugh G, Weiss HA. Rehman AM, et al. BMJ Open. 2020 Dec 2;10(12):e039546. doi: 10.1136/bmjopen-2020-039546. BMJ Open. 2020. PMID: 33268410 Free PMC article. Clinical Trial. - Effectiveness and safety of Jiuwei Zhenxin granules for treating generalized anxiety disorder: A randomized controlled trial.
Wang X, Chen P, Shi S, Chen W, Zhang H, Tang R, Wu Z, Li Y, Wu J, Zong L, Ji L, Feng P, Li J. Wang X, et al. Front Psychiatry. 2022 Oct 4;13:898683. doi: 10.3389/fpsyt.2022.898683. eCollection 2022. Front Psychiatry. 2022. PMID: 36267853 Free PMC article.
References
- Newell DJ. Intention-to-treat analysis: implications for quantitative and qualitative research. Int J Epidemiol 1992;21:837-41. - PubMed
- Tierney JF, Stewart LA. Investigating patient exclusion bias in meta-analysis. Int J Epidemiol 2005;34:79-87. - PubMed
- Higgins J, Green S. Intention-to-treat issues. In: Cochrane Collaboration. Cochrane handbook for systematic reviews on interventions. Cochrane Collaboration, 2008.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources