Selective neuronal vulnerability to oxidative stress in the brain - PubMed (original) (raw)
Selective neuronal vulnerability to oxidative stress in the brain
Xinkun Wang et al. Front Aging Neurosci. 2010.
Abstract
Oxidative stress (OS), caused by the imbalance between the generation and detoxification of reactive oxygen and nitrogen species (ROS/RNS), plays an important role in brain aging, neurodegenerative diseases, and other related adverse conditions, such as ischemia. While ROS/RNS serve as signaling molecules at physiological levels, an excessive amount of these molecules leads to oxidative modification and, therefore, dysfunction of proteins, nucleic acids, and lipids. The response of neurons to this pervasive stress, however, is not uniform in the brain. While many brain neurons can cope with a rise in OS, there are select populations of neurons in the brain that are vulnerable. Because of their selective vulnerability, these neurons are usually the first to exhibit functional decline and cell death during normal aging, or in age-associated neurodegenerative diseases, such as Alzheimer's disease. Understanding the molecular and cellular mechanisms of selective neuronal vulnerability (SNV) to OS is important in the development of future intervention approaches to protect such vulnerable neurons from the stresses of the aging process and the pathological states that lead to neurodegeneration. In this review, the currently known molecular and cellular factors that contribute to SNV to OS are summarized. Included among the major underlying factors are high intrinsic OS, high demand for ROS/RNS-based signaling, low ATP production, mitochondrial dysfunction, and high inflammatory response in vulnerable neurons. The contribution to the selective vulnerability of neurons to OS by other intrinsic or extrinsic factors, such as deficient DNA damage repair, low calcium-buffering capacity, and glutamate excitotoxicity, are also discussed.
Keywords: aging; energy metabolism; glia; mitochondria; neurodegeneration; oxidative stress; selective neuronal vulnerability; signaling.
Figures
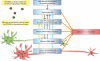
Figure 1
Molecular and cellular factors that contribute to the selective vulnerability of neurons to oxidative stress. ROS/RNS can serve as signaling molecules while they cause damages to bio-molecules at increased levels. Neurons in different parts of the brain have differential needs for ROS/RNS as signaling molecules, with some neurons (such as those in the hippocampal CA1 region) having higher demand than others. However, due to the duality of ROS/RNS, the high demand for these highly reactive species may lead to intrinsically high OS in some neurons, which can make them selectively vulnerable when facing increased stress. Vulnerable neurons are also characterized by low ATP production and mitochondrial dysfunction, possibly because of the high OS state in these neurons, and other factors such as calcium dysregulation. Low ATP production can affect DNA repair, which, when combined with high DNA oxidation, can cause change of genomic activity and decreased metabolic activity in mitochondria. Functional genomics studies also suggest existence of chronic inflammatory response in vulnerable neurons, which can further elevate OS within them. Calcium dysregulation and glutamate hyperactivity are closely connected to OS generation and underlie many adverse conditions that are characterized by SNV. There is emerging evidence that directly connects these factors, such as low calcium-buffering capacity and glutamate-mediated selective neurodegeneration, to the selective vulnerability of neurons. The colored arrows that link these mechanistic factors in the figure denote direct relationships between them. In addition, vulnerable neurons tend to be large in size, with long projecting axons to their targets.
Similar articles
- Genomic and biochemical approaches in the discovery of mechanisms for selective neuronal vulnerability to oxidative stress.
Wang X, Zaidi A, Pal R, Garrett AS, Braceras R, Chen XW, Michaelis ML, Michaelis EK. Wang X, et al. BMC Neurosci. 2009 Feb 19;10:12. doi: 10.1186/1471-2202-10-12. BMC Neurosci. 2009. PMID: 19228403 Free PMC article. - Oxidative stress and protein aggregation during biological aging.
Squier TC. Squier TC. Exp Gerontol. 2001 Sep;36(9):1539-50. doi: 10.1016/s0531-5565(01)00139-5. Exp Gerontol. 2001. PMID: 11525876 Review. - Functional genomics of brain aging and Alzheimer's disease: focus on selective neuronal vulnerability.
Wang X, Michaelis ML, Michaelis EK. Wang X, et al. Curr Genomics. 2010 Dec;11(8):618-33. doi: 10.2174/138920210793360943. Curr Genomics. 2010. PMID: 21629439 Free PMC article. - Dual effects of antioxidants in neurodegeneration: direct neuroprotection against oxidative stress and indirect protection via suppression of glia-mediated inflammation.
Wang JY, Wen LL, Huang YN, Chen YT, Ku MC. Wang JY, et al. Curr Pharm Des. 2006;12(27):3521-33. doi: 10.2174/138161206778343109. Curr Pharm Des. 2006. PMID: 17017945 Review. - Oxidative Stress: A Key Modulator in Neurodegenerative Diseases.
Singh A, Kukreti R, Saso L, Kukreti S. Singh A, et al. Molecules. 2019 Apr 22;24(8):1583. doi: 10.3390/molecules24081583. Molecules. 2019. PMID: 31013638 Free PMC article. Review.
Cited by
- Neuroprotective effect of phospholipase A2 from Malaysian Naja sumatrana venom against H2O2-induced cell damage and apoptosis.
Abdullah NAH, Sainik NQAV, Esa E, Muhamad Hendri NA, Ahmad Rusmili MR, Hodgson WC, Shaikh MF, Othman I. Abdullah NAH, et al. Front Pharmacol. 2022 Oct 14;13:935418. doi: 10.3389/fphar.2022.935418. eCollection 2022. Front Pharmacol. 2022. PMID: 36313292 Free PMC article. - Inhibiting amyloid beta (1-42) peptide-induced mitochondrial dysfunction prevents the degradation of synaptic proteins in the entorhinal cortex.
Olajide OJ, La Rue C, Bergdahl A, Chapman CA. Olajide OJ, et al. Front Aging Neurosci. 2022 Oct 6;14:960314. doi: 10.3389/fnagi.2022.960314. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36275011 Free PMC article. - L-carnitine: A novel approach in management of acute cholinesterase inhibitor insecticide poisoning.
Ragab HE, El-Banna A, Elshaer NS, Azzaz O. Ragab HE, et al. Toxicol Res (Camb). 2024 Jul 10;13(4):tfae104. doi: 10.1093/toxres/tfae104. eCollection 2024 Aug. Toxicol Res (Camb). 2024. PMID: 38993484 - Caffeine Administration Mitigates Chronic Stress-Induced Behavioral Deficits, Neurochemical Alterations, and Glial Disruptions in Rats.
Okeowo OM, Oke OO, David GO, Ijomone OM. Okeowo OM, et al. Brain Sci. 2023 Nov 30;13(12):1663. doi: 10.3390/brainsci13121663. Brain Sci. 2023. PMID: 38137111 Free PMC article. - Differential vulnerability of substantia nigra and corpus striatum to oxidative insult induced by reduced dietary levels of essential fatty acids.
Cardoso HD, Passos PP, Lagranha CJ, Ferraz AC, Santos Júnior EF, Oliveira RS, Oliveira PE, Santos Rde C, Santana DF, Borba JM, Rocha-de-Melo AP, Guedes RC, Navarro DM, Santos GK, Borner R, Picanço-Diniz CW, Beltrão EI, Silva JF, Rodrigues MC, Andrade da Costa BL. Cardoso HD, et al. Front Hum Neurosci. 2012 Aug 30;6:249. doi: 10.3389/fnhum.2012.00249. eCollection 2012. Front Hum Neurosci. 2012. PMID: 22969716 Free PMC article.
References
- Atwood C. S., Scarpa R. C., Huang X., Moir R. D., Jones W. D., Fairlie D. P., Tanzi R. E., Bush A. I. (2000). Characterization of copper interactions with alzheimer amyloid beta peptides: identification of an attomolar-affinity copper binding site on amyloid beta1-42. J. Neurochem. 75, 1219–123310.1046/j.1471-4159.2000.0751219.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases