Recent advances in neuroblastoma - PubMed (original) (raw)
Review
Recent advances in neuroblastoma
John M Maris. N Engl J Med. 2010.
No abstract available
Conflict of interest statement
Dr. Maris reports receiving grants from GlaxoSmithKline and Merck and being a named investigator on two pending U.S. patents for methods to identify neuroblastomas, for which he has not received royalties. No other potential conflict of interest relevant to this article was reported.
Figures
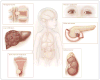
Figure 1. Clinical Presentations of Neuroblastoma
Neuroblastoma is a childhood cancer that is diagnosed at a median age of about 17 months. Tumors can arise anywhere along the sympathetic nervous system, with the majority occurring in the adrenal medulla. Primary tumors in the neck or upper chest can cause Horner’s syndrome (ptosis, miosis, and anhidrosis). Tumors along the spinal column can expand through the intraforaminal spaces and cause cord compression, with resulting paralysis. Although many lower-stage neuroblastomas are encapsulated and can be surgically excised with little chance of complications, higher-stage tumors often infiltrate local organ structures, surround critical nerves and vessels such as the celiac axis, and are largely unresectable at the time of diagnosis. Neuroblastomas typically metastasize to regional lymph nodes and to the bone marrow by means of the hematopoietic system. Tumor cells metastatic to marrow can infiltrate cortical bone. Neuroblastomas also can metastasize to the liver, most notably in patients with stage 4S tumors, in whom involvement can be extensive; however, transient and complete regression often occurs with no intervention other than supportive care.
Figure 2. Model of Genetic Susceptibility to Neuroblastoma
The y axis indicates the theoretical relative risk of neuroblastoma, and the x axis indicates the number of known and theoretical susceptibility alleles. A genetic threshold for the development of disease has been postulated, and malignant transformation is probably modified by interactions related to environmental exposure. A mutation in the ALK or PHOX2B gene results in a single, highly penetrant risk allele that allows developing neuroblastic tissue to meet or exceed this threshold for malignant transformation. These types of mutations are powerful enough to permit neuroblastoma to occur within families as a mendelian trait. On the other hand, there are multiple common DNA variations (polymorphisms) in a large number of genes that cooperate to reach this threshold in patients without ALK or PHOX2B mutations. For these sporadic cases of neuroblastoma, an excessive inheritance of “risk” variants has been postulated that increases susceptibility to the disease. Discovered susceptibility genes include FLJ22536, BARD1, and NBPF23. The total number of susceptibility loci is not currently known, nor is it known whether these polymorphisms act in an additive or synergistic (epistatic) fashion.
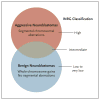
Figure 3. Genomic Basis of Neuroblastoma Risk Groups
Two broad neuroblastoma phenotypes — aggressive and benign — are seen clinically, with the latter showing a high propensity for spontaneous regression or differentiation. These two groups are largely identifiable at a chromosomal level by the presence of segmental aberrations (translocations, amplifications, and deletions) in the more aggressive cases and by whole-chromosome gains in the more benign cases. Thus, the International Neuroblastoma Risk Group (INRG) classification is related to these chromosomal alterations, but the current system is imprecise, since the intermediate group in particular remains poorly defined. Current investigation is focused on the identification of molecular predictors of outcome in the high-risk group (as well as in patients with aggressive neuroblastomas masquerading as more benign forms of the disease).
Similar articles
- Treatment and outcomes of patients with relapsed, high-risk neuroblastoma: results of German trials.
Simon T, Berthold F, Borkhardt A, Kremens B, De Carolis B, Hero B. Simon T, et al. Pediatr Blood Cancer. 2011 Apr;56(4):578-83. doi: 10.1002/pbc.22693. Epub 2010 Dec 9. Pediatr Blood Cancer. 2011. PMID: 21298742 - [Efficacy of chemotherapy regimen using ifosphamide and carboplatin on recurrent or refractory neuroblastoma].
Zhen ZJ, Sun XF, Xia Y, Wang ZH, Ling JY. Zhen ZJ, et al. Ai Zheng. 2006 Dec;25(12):1550-2. Ai Zheng. 2006. PMID: 17166384 Chinese. - Intensified chemotherapy increases the survival rates in patients with stage 4 neuroblastoma with MYCN amplification.
Kaneko M, Tsuchida Y, Mugishima H, Ohnuma N, Yamamoto K, Kawa K, Iwafuchi M, Sawada T, Suita S. Kaneko M, et al. J Pediatr Hematol Oncol. 2002 Nov;24(8):613-21. doi: 10.1097/00043426-200211000-00004. J Pediatr Hematol Oncol. 2002. PMID: 12439032 - High-dose chemotherapy and autologous haematopoietic stem cell rescue for children with high-risk neuroblastoma.
Yalçin B, Kremer LC, van Dalen EC. Yalçin B, et al. Cochrane Database Syst Rev. 2015 Oct 5;2015(10):CD006301. doi: 10.1002/14651858.CD006301.pub4. Cochrane Database Syst Rev. 2015. PMID: 26436598 Free PMC article. Review. - High-dose chemotherapy and autologous haematopoietic stem cell rescue for children with high-risk neuroblastoma.
Yalçin B, Kremer LC, Caron HN, van Dalen EC. Yalçin B, et al. Cochrane Database Syst Rev. 2013 Aug 22;(8):CD006301. doi: 10.1002/14651858.CD006301.pub3. Cochrane Database Syst Rev. 2013. PMID: 23970444 Updated. Review.
Cited by
- Identification of novel markers for neuroblastoma immunoclustering using machine learning.
Zhang L, Li H, Sun F, Wu Q, Jin L, Xu A, Chen J, Yang R. Zhang L, et al. Front Immunol. 2024 Nov 4;15:1446273. doi: 10.3389/fimmu.2024.1446273. eCollection 2024. Front Immunol. 2024. PMID: 39559348 Free PMC article. - Combinatorial immunotherapy of anti-MCAM CAR-modified expanded natural killer cells and NKTR-255 against neuroblastoma.
Luo W, Gardenswartz A, Hoang H, Chu Y, Tian M, Liao Y, Ayello J, Rosenblum JM, Mo X, Marcondes AM, Overwijk WW, Cripe TP, Lee DA, Cairo MS. Luo W, et al. Mol Ther Oncol. 2024 Oct 18;32(4):200894. doi: 10.1016/j.omton.2024.200894. eCollection 2024 Dec 19. Mol Ther Oncol. 2024. PMID: 39554906 Free PMC article. - Segmental chromosome aberrations as a prognostic factor of neuroblastoma: a meta-analysis and systematic review.
Geng J, Wang X, Zhao L, Zhang J, Niu H. Geng J, et al. Transl Pediatr. 2024 Oct 1;13(10):1789-1798. doi: 10.21037/tp-24-200. Epub 2024 Oct 28. Transl Pediatr. 2024. PMID: 39524401 Free PMC article. - Immunomodulatory properties of extracellular vesicles isolated from bone marrow of patients with neuroblastoma: role of PD-L1 and HLA-G.
Marimpietri D, Corrias MV, Tripodi G, Gramignoli R, Airoldi I, Morandi F. Marimpietri D, et al. Front Immunol. 2024 Oct 24;15:1469771. doi: 10.3389/fimmu.2024.1469771. eCollection 2024. Front Immunol. 2024. PMID: 39512342 Free PMC article. - Recommendations for the use of nuclear medicine imaging in patients with neuroblastoma.
Sánchez-Vañó R, Balaguer J, Borrego-Dorado I, Esteban-Figueruelo A, Gámez C, Hladun R, López-Almaraz R, Llempén ML, Rodado S, Rubio-Aparicio PM. Sánchez-Vañó R, et al. Clin Transl Oncol. 2024 Nov 7. doi: 10.1007/s12094-024-03755-3. Online ahead of print. Clin Transl Oncol. 2024. PMID: 39508974 Review.
References
- Hoehner JC, Gestblom C, Hedborg F, Sandstedt B, Olsen L, Pahlman S. A developmental model of neuroblastoma: differentiating stroma-poor tumors’ progress along an extra-adrenal chromaffin lineage. Lab Invest. 1996;75:659–75. - PubMed
- London WB, Castleberry RP, Matthay KK, et al. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children’s Oncology Group. J Clin Oncol. 2005;23:6459–65. - PubMed
- Ries LAG, Smith MA, Gurney JG, et al. Cancer incidence and survival among children and adolescents: United States SEER program 1975–1995. Bethesda, MD: National Cancer Institute; 1999. (NIH publication no. 99–4649.)
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical