The habenula: from stress evasion to value-based decision-making - PubMed (original) (raw)
Review
The habenula: from stress evasion to value-based decision-making
Okihide Hikosaka. Nat Rev Neurosci. 2010 Jul.
Abstract
Surviving in a world with hidden rewards and dangers requires choosing the appropriate behaviours. Recent discoveries indicate that the habenula plays a prominent part in such behavioural choice through its effects on neuromodulator systems, in particular the dopamine and serotonin systems. By inhibiting dopamine-releasing neurons, habenula activation leads to the suppression of motor behaviour when an animal fails to obtain a reward or anticipates an aversive outcome. Moreover, the habenula is involved in behavioural responses to pain, stress, anxiety, sleep and reward, and its dysfunction is associated with depression, schizophrenia and drug-induced psychosis. As a highly conserved structure in the brain, the habenula provides a fundamental mechanism for both survival and decision-making.
Figures
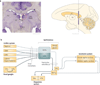
Figure 1. Anatomy of the habenula
a | The habenula in the rhesus monkey. A coronal histological section (scale: 1 mm × 5) shows the habenula (indicated by the red circle). The medially located dark region corresponds to the medial habenula (MHb) and the lateral part corresponds to the lateral habenula (LHb). The violet line in the diagram of the monkey brain (viewed from the mesial side) corresponds to the vertical extent of the cross-section view of the habenula (scale: 5 mm × 2). The location of the habenula is also indicated in the diagram of the monkey brain (indicated by the orange circle). C, caudate nucleus; cc, corpus callosum; hc, habenular commissure; IC, inferior colliculus; MD, mediodorsal nucleus of the thalamus; N3, oculomotor nucleus; SC, superior colliculus; pc, posterior commissure; PT, pretectum; Pul, pulvinar; Th, thalamus. b | Afferent and efferent connections of the habenula. The MHb, LHb and pineal gland are collectively called the epithalamus. The MHb receives inputs mainly from the limbic system and sends outputs to the interpeduncular nucleus (IPN), which projects to the raphe nuclei. The LHb receives inputs mainly from the basal ganglia and sends outputs to the brain structures that contain dopaminergic neurons and serotonergic neurons, partly through the rostromedial tegmental nucleus (RMTg). Direct connections to dopaminergic and serotonergic neurons are not shown. The role of the RMTg for serotonergic neurons is less clear (indicated by the dashed lines). Light and dark grey lines indicate the axonal connections associated with the MHb and LHb, respectively. Many other connections are not shown, including reverse connections (for example, from the dorsal raphe nucleus and the ventral tegmental area (VTA) to the LHb). CPu, caudate and putamen; DBB, diagonal band of Broca; GPb, border region of the globus pallidus; LPO, lateral preoptic area; SNc, substantia nigra pars compacta.
Figure 2. Proposed common mechanisms for the diverse functions of the habenula
The habenula is equipped with mechanisms by which body movements can be suppressed. How the motor suppression mechanisms are used depends on the information that is fed to the habenula. a | A phylogenetically old input to the habenula derives from the pineal gland, which encodes light–dark changes and regulates circadian rhythms. The habenula, which has reciprocal connections with the pineal glad, controls the level of motor activity according to sleep–wake states through its polysynaptic connections to neural circuits in the brainstem. b | A second input to the habenula derives from the basal ganglia, which encodes failure or punishment resulting from a motor action. Based on this input, the habenula inhibits dopamine neurons in the ventral tegmental area (VTA) and substantia nigra pars compacta (SNc). This results in suppression of the motor action. c | A third input to the habenula derives from the limbic system, which conveys information regarding aversive, painful or stressful events. In this case the habenula inhibits both serotonin and dopamine neurons and this results in a general suppression of body movement.
Figure 3. Role of the habenula in value-based decision-making
This circuit is an extended form of the dopamine-mediated circuit shown in FIG. 2b. Input from the basal ganglia to the habenula, specifically the lateral habenula (LHb), derives from the border region of the internal segment of the globus pallidus (GPb), which receives inputs from the striatum — presumably the ‘striosome’ subterritory of the striatum (Striatum-S). Based on the basal ganglia input, the habenula influences dopamine neurons via inhibitory neurons in the rostromedial tegmental nucleus (RMTg). Negative reward prediction errors are encoded by an excitation of LHb neurons and consequently an inhibition of dopamine neurons. Positive reward prediction errors are encoded by an inhibition of LHb neurons and an excitation of dopamine neurons. Such bidirectional modulation of dopamine neurons contributes to the suppression of the to-be-less-rewarded motor action and the facilitation of the to-be-more-rewarded motor action. These effects on motor action are mediated by the innervation of the ‘matrix’ subterritory of the striatum (Striatum-M) by dopamine neurons in the ventral tegmental area (VTA) and substatia nigra pars compacta (SNc), and subsequently the innervation of the output region of the basal ganglia, that is, the substantia nigra pars reticulata (SNr) and the internal segment of the globus pallidus (GPi).
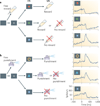
Figure 4. The lateral habenula–dopamine system for modulation of saccadic eye movements
a | Visual saccade task with position-biased reward outcomes. In one block of 20–30 trials, saccades in one direction (to the right) were rewarded, whereas saccades in the other direction (to the left) were not rewarded. The position–reward contingency was reversed in the next block without warning (not shown). In both blocks, after each correct trial a tone indicated that a correct response (that is, a saccade in the correct direction) had been made and a juice reward was delivered simultaneously with the tone. b | In each block, saccades to the rewarded position (shown by the red circles) became quicker (shorter latencies) whereas saccades to the unrewarded position (shown by the blue squares) became slower (longer latencies). c | The reward-indicating saccade target induced an inhibition in lateral habenula (LHb) neurons (top) and an excitation in dopamine (DA) neurons in the substantia nigra pars compacta (SNc) (bottom; shown by the red traces); the no-reward-indicating target induced an excitation in LHb neurons and an inhibition in dopamine neurons (shown by the blue traces). The LHb and dopamine neurons also responded strongly to the outcomes in the first trials of a block when the outcomes were unexpected (dashed red and blue traces). Figure is modified, with permission, from Nature REF. © (2007) Macmillan Publishers Ltd. All rights reserved.
Figure 5. The lateral habenula encodes motivational values
A Pavlovian procedure with two distinct contexts (a and b). a | An appetitive block in which juice served as a reward. Three conditioned stimuli (CSs) were associated with the reward and indicated 100%, 50% and 0% probability of receiving the reward, respectively. b | An aversive block in which an air puff was delivered as a punishment. Three CSs were associated with the punishment and indicated 100%, 50% and 0% probability of receiving the punishment, respectively. Each trial of each block started after the presentation of a timing cue (a small central spot) on the screen. After 1 s, 1 of the 3 CSs was presented pseudo-randomly. After 1.5 s, the CS disappeared and the unconditioned stimulus (US; juice or air puff) was delivered. In addition to the cued trials, uncued trials were included in which a juice reward alone (free reward) was delivered during the appetitive block and an air puff alone (free punishment) was delivered during the aversive block. The activity of a lateral habenula neuron in response to the CSs is shown by the blue traces in the right hand column. Spike density functions are aligned by the onset of each CS (shown by the dashed black lines). Figure is modified, with permission, from Nature Neuroscience REF. © (2009) Macmillan Publishers Ltd. All rights reserved.
Similar articles
- What's better for me? Fundamental role for lateral habenula in promoting subjective decision biases.
Stopper CM, Floresco SB. Stopper CM, et al. Nat Neurosci. 2014 Jan;17(1):33-5. doi: 10.1038/nn.3587. Epub 2013 Nov 24. Nat Neurosci. 2014. PMID: 24270185 Free PMC article. - Translating the Habenula-From Rodents to Humans.
Boulos LJ, Darcq E, Kieffer BL. Boulos LJ, et al. Biol Psychiatry. 2017 Feb 15;81(4):296-305. doi: 10.1016/j.biopsych.2016.06.003. Epub 2016 Jun 7. Biol Psychiatry. 2017. PMID: 27527822 Free PMC article. Review. - Reward and avoidance learning in the context of aversive environments and possible implications for depressive symptoms.
Sebold M, Garbusow M, Jetzschmann P, Schad DJ, Nebe S, Schlagenhauf F, Heinz A, Rapp M, Romanczuk-Seiferth N. Sebold M, et al. Psychopharmacology (Berl). 2019 Aug;236(8):2437-2449. doi: 10.1007/s00213-019-05299-9. Epub 2019 Jun 28. Psychopharmacology (Berl). 2019. PMID: 31254091 Free PMC article. Clinical Trial. - A hypothalamus-habenula circuit controls aversion.
Lazaridis I, Tzortzi O, Weglage M, Märtin A, Xuan Y, Parent M, Johansson Y, Fuzik J, Fürth D, Fenno LE, Ramakrishnan C, Silberberg G, Deisseroth K, Carlén M, Meletis K. Lazaridis I, et al. Mol Psychiatry. 2019 Sep;24(9):1351-1368. doi: 10.1038/s41380-019-0369-5. Epub 2019 Feb 12. Mol Psychiatry. 2019. PMID: 30755721 Free PMC article. - Reward processing by the lateral habenula in normal and depressive behaviors.
Proulx CD, Hikosaka O, Malinow R. Proulx CD, et al. Nat Neurosci. 2014 Sep;17(9):1146-52. doi: 10.1038/nn.3779. Nat Neurosci. 2014. PMID: 25157511 Free PMC article. Review.
Cited by
- Lateral Habenula Mediates Defensive Responses Only When Threat and Safety Memories Are in Conflict.
Velazquez-Hernandez G, Sotres-Bayon F. Velazquez-Hernandez G, et al. eNeuro. 2021 Apr 19;8(2):ENEURO.0482-20.2021. doi: 10.1523/ENEURO.0482-20.2021. Print 2021 Mar-Apr. eNeuro. 2021. PMID: 33712440 Free PMC article. - Distinct requirements for Wntless in habenular development.
Kuan YS, Roberson S, Akitake CM, Fortuno L, Gamse J, Moens C, Halpern ME. Kuan YS, et al. Dev Biol. 2015 Oct 15;406(2):117-128. doi: 10.1016/j.ydbio.2015.06.006. Epub 2015 Jun 23. Dev Biol. 2015. PMID: 26116173 Free PMC article. - Excitatory Transmission to the Lateral Habenula Is Critical for Encoding and Retrieval of Spatial Memory.
Mathis V, Cosquer B, Avallone M, Cassel JC, Lecourtier L. Mathis V, et al. Neuropsychopharmacology. 2015 Nov;40(12):2843-51. doi: 10.1038/npp.2015.140. Epub 2015 May 14. Neuropsychopharmacology. 2015. PMID: 25971591 Free PMC article. - The role of medial prefrontal cortex in memory and decision making.
Euston DR, Gruber AJ, McNaughton BL. Euston DR, et al. Neuron. 2012 Dec 20;76(6):1057-70. doi: 10.1016/j.neuron.2012.12.002. Neuron. 2012. PMID: 23259943 Free PMC article. Review. - Exploration of the white matter bundles connected to the pineal gland: A DTI study.
Kiani P, Hassanzadeh G, Jameie SB, Batouli SAH. Kiani P, et al. Surg Radiol Anat. 2024 Oct;46(10):1571-1584. doi: 10.1007/s00276-024-03445-3. Epub 2024 Aug 5. Surg Radiol Anat. 2024. PMID: 39102045
References
- Dadda M, Domenichini A, Piffer L, Argenton F, Bisazza A. Early differences in epithalamic left–right asymmetry influence lateralization and personality of adult zebrafish. Behav. Brain Res. 2010;206:208–215. - PubMed
- Guglielmotti V, Cristino L. The interplay between the pineal complex and the habenular nuclei in lower vertebrates in the context of the evolution of cerebral asymmetry. Brain Res. Bull. 2006;69:475–488. - PubMed
- Ronnekleiv OK, Moller M. Brain-pineal nervous connections in the rat: an ultrastructure study following habenular lesion. Exp. Brain Res. 1979;37:551–562. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials