Regulation of amyloid precursor protein processing by the Beclin 1 complex - PubMed (original) (raw)
Regulation of amyloid precursor protein processing by the Beclin 1 complex
Philipp A Jaeger et al. PLoS One. 2010.
Abstract
Autophagy is an intracellular degradation pathway that functions in protein and organelle turnover in response to starvation and cellular stress. Autophagy is initiated by the formation of a complex containing Beclin 1 (BECN1) and its binding partner Phosphoinositide-3-kinase, class 3 (PIK3C3). Recently, BECN1 deficiency was shown to enhance the pathology of a mouse model of Alzheimer Disease (AD). However, the mechanism by which BECN1 or autophagy mediate these effects are unknown. Here, we report that the levels of Amyloid precursor protein (APP) and its metabolites can be reduced through autophagy activation, indicating that they are a substrate for autophagy. Furthermore, we find that knockdown of Becn1 in cell culture increases the levels of APP and its metabolites. Accumulation of APP and APP C-terminal fragments (APP-CTF) are accompanied by impaired autophagosomal clearance. Pharmacological inhibition of autophagosomal-lysosomal degradation causes a comparable accumulation of APP and APP-metabolites in autophagosomes. Becn1 reduction in cell culture leads to lower levels of its binding partner Pik3c3 and increased presence of Microtubule-associated protein 1, light chain 3 (LC3). Overexpression of Becn1, on the other hand, reduces cellular APP levels. In line with these observations, we detected less BECN1 and PIK3C3 but more LC3 protein in brains of AD patients. We conclude that BECN1 regulates APP processing and turnover. BECN1 is involved in autophagy initiation and autophagosome clearance. Accordingly, BECN1 deficiency disrupts cellular autophagy and autophagosomal-lysosomal degradation and alters APP metabolism. Together, our findings suggest that autophagy and the BECN1-PIK3C3 complex regulate APP processing and play an important role in AD pathology.
Conflict of interest statement
Competing Interests: The authors received partial funding from Biogen Idec. However, no competing interests exist, and all data and materials are available to the scientific community in adherence to all the PLoS ONE policies.
Figures
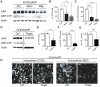
Figure 1. Activation of autophagy promotes APP, APP-CTF, and Aβ degradation.
A–C. B103/hAPP cells were left untreated (Ctrl), starved for 90 min in HANKS solution (Starv), or treated with 100 nM rapamycin in DMEM (Rap) for 90 min. Western blots (A) and quantification (B, C) of RIPA cell lysates probed with the CT15 antibody recognizing full-length APP and APP-CTFs and with an actin antibody as a control for loading. D–F. CHO/hAPP cells were left untreated (Ctrl) or treated for 12 hrs with 3 µM thapsigargin (Thaps) in DMEM/10%FBS. Western blots (D) and quantification (E, F) of RIPA cell lysates probed with antibodies as in A. (Data from the same blot. The vertical line indicates removal of three lanes not part of this experiment.) G. Secretion of Aβ into the cell supernatant was measured by ELISA (12 hrs/1 µM Thaps) H. Epifluorescence microscopy images of CHO/hAPP cells treated as in D, permeabilized with Tween and stained with antibody CT20 to label all cellular APP, or not permeabilized and stained with antibody 8E5 which recognizes the ectodomain of APP at the cell surface (scale bar represents 25 µm). Bars are mean ± SEM from triplicate cultures of at least two independent experiments. * p<0.05, ** p<0.01, *** p<0.001 by unpaired Student's t test.
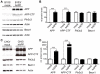
Figure 2. Becn1 knockdown increases APP, APP-like proteins, APP-CTFs and Aβ.
A–B. B103/hAPP cells were treated with Becn1 siRNA for 48–72 hrs. Cells were left untreated (U), treated with transfection reagent alone (no RNA), treated with scrambled siRNA (Ctrl siRNA [C]), or treated with Becn1 siRNA (Becn1 siRNA [B]). Western blots (A) of RIPA cell lysates were probed with a Becn1 antibody, the CT15 APP antibody, and with an actin antibody as a control for loading. For quantification see Fig, S2. (Data from two blots with identical exposure times. Blot border indicated by vertical black line.) Total Aβ1-x concentrations measured by ELISA in cell culture supernatant from the same cells at 72 hrs (B). C–D. CHO/hAPP cells were treated with Becn1 siRNA for 48 hrs. Western blots (C) and quantification (D) of RIPA cell lysates that were probed with a Becn1 antibody, the CT15 APP antibody, and with an actin antibody as a control for loading. E–F. CHO/APLP1 cells were treated with Becn1 siRNA for 48 hrs. Western blots (E) and quantification (F) of RIPA cell lysates that were probed with a Becn1 antibody, an APLP1 antibody, and with an actin antibody as a control for loading. G–H. CHO/APLP2 cells were treated with Becn1 siRNA for 48 hrs. Western blots (G) and quantification (H) of RIPA cell lysates that were probed with a Becn1 antibody, an APLP2 antibody, and with an actin antibody as a control for loading. J. Levels of APP mRNA were compared by qRT-PCR in scrambled [C] or Becn1 [B] siRNA treated B103/hAPP cells. K–L. CHO/hAPP cells were treated with either GFP lentivirus or Becn1 shRNA-GFP lentivirus. Quantification of the relative APP immunofluorescence (K) and epifluorescence microscopy (L) of GFP lentivirus or Becn1 shRNA-GFP lentivirus treated permeabilized CHO/hAPP cells, probed with DAPI and CT20 APP antibody (scale bar represents 10 µm). M. Inhibition of γ-secretase activity through 100 nM DAPT treatment had no significant effect on APP levels and an additive effect on APP-CTF accumulation with Becn1 shLV treatment. Bars are mean ± SEM from duplicate/triplicate cultures of at least two independent experiments. * p<0.05, ** p<0.01, *** p<0.001 by unpaired Student's t test.
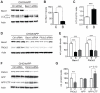
Figure 3. Overexpression of APP does not change Becn1 or Pik3c3 protein levels.
A–B. B103 cells were stably transfected with an empty plasmid (mock) or a hAPP encoding plasmid. Western blots (A) and quantification (B) of RIPA cell lysates that were probed with the CT15 APP, a Becn1, and a Pik3c3 antibody. An actin antibody was used as a loading control. C–D. CHO cells were stably transfected with an empty plasmid (mock) or a hAPP encoding plasmid. Western blots (C) and quantification (D) of RIPA cell lysates that were probed with the CT15 APP, a Becn1, and a Pik3c3 antibody. Actin antibody was used as a loading control. Bars are mean ± SEM from triplicate cultures of at least two independent experiments. * p<0.05, ** p<0.01, *** p<0.001 by unpaired Student's t test.
Figure 4. Reduction of Becn1 impairs degradation of autophagosomes and reduces Pik3c3 levels.
A–C. CHO/hAPP cells were treated with Becn1 siRNA for 48 h. Western blots (A) of RIPA cell lysates were probed with a Becn1 and LC3 antibody. An actin antibody was used as a loading control. Quantification (B) of the Becn1 band intensity and the ratio of LC3-II to LC3-I (C). D–E. CHO/hAPP cells were treated with Becn1 and Pik3c3 siRNA for 48 h. Western blots (D) and quantification (E) of RIPA cell lysates that were probed with a Becn1 and Pik3c3 antibody. An actin antibody was used as a loading control. F–G. CHO/hAPP cells were treated with Pik3c3 siRNA for 48 h. Western blots (F) and quantification (G) of RIPA cell lysates that were probed with the CT15 APP antibody and with an actin antibody as a control for loading. Bars are mean ± SEM from triplicate cultures of at least two independent experiments. * p<0.05, ** p<0.01, *** p<0.001 by unpaired Student's t test.
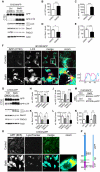
Figure 5. Inhibition of autophagosomal turnover leads to a reduction in Becn1 and Pik3c3 levels.
A–E. B103/hAPP cells were treated with vehicle (DMSO) or 50 nM BafA for 24 hrs to inhibit autophagosomal degradation. Western blots (A) and quantification (B–E) of RIPA cell lysates that were probed with CT15 APP, LC3, Becn1, and Pik3c3 antibody. An actin antibody was used as a loading control. F. Confocal microscopy of B103/hAPP cells treated with vehicle (DMSO) or 100 nM BafA for 24 hrs. Cells were stained with CT20 APP antibody (magenta) and LC3 antibody (cyan). Co-localization is indicated in yellow. Arrowheads indicate LC3 positive APP containing vesicles. The arrow indicates an APP containing LC3 negative vesicle (scale bar represents 10 µm). The line indicates cross-section. Cyan line in the cross-section represents APP intensity, magenta line represents LC3 intensity (AU). G–L. CHO/hAPP cells were treated with vehicle (DMSO), 50 nM, or 100 nM BafA (WB data not shown) for 24 hrs. Western blots (G) and quantification (H–L) of RIPA cell lysates that were probed with the CT15 APP, LC3, Becn1, and Pik3c3 antibody. An actin antibody was used as a loading control. M–N. BafA and CQ treatment cause increased APP processing which in turn leads to elevated levels of secreted APP (sAPP) in the cell supernatant (M). This is quantified in (N). O. Epifluorescence microscopy of CHO/hAPP cells treated with vehicle (DMSO) or 100 nM BafA for 12 hrs. Cells were stained with the 8E5 APP antibody (magenta) and LysoTracker (cyan). Co-localization is indicated in yellow (scale bar represents 25 µm). P. Schematic representation of the APP antibody epitopes. Bars are mean ± SEM from triplicate cultures of at least two independent experiments. * p<0.05, ** p<0.01, *** p<0.001 by unpaired Student's t test.
Figure 6. Becn1 overexpression reduces APP immunoreactivity.
A. CHO/hAPP cells were transduced with either a GFP LV (GFP control) or a mBecn1 LV (Becn1 o.e.). Epifluorescence microscopy was performed after staining with Becn1 and APP CT15 antibodies (Scale bar represents 25 µm). GFP LV transduced cells show very faint Becn1 immunoreactivity, while Becn1 LV transduced cells exhibit a range of Becn1 signal intensity. No GFP signal is present in the Becn1 LV cells. A random selection of cells (N = 214) was picked from the GFP LV cells and the Becn1 LV cells. The Becn1 LV cells were randomly selected in both, the APP (yellow outline) and the Becn1 (red outline) channel. B. Relative immunofluorescence of the selected cells (AU). They can be divided in low, medium, and high Becn1 expressing cells. C. Quantification of the relative APP immunofluorescence in the three cohorts. Medium Becn1 overexpression leads to a significant reduction in APP levels. Medians were compared by Man-Whitney U test. * p<0.05, ** p<0.01, *** p<0.001
Figure 7. AD brains have less BECN1 and PIK3C3 and more LC3.
A–H. Comparison of protein levels in frontal cortex (gray matter) from AD brains and age matched, non-demented, non-pathological controls. Western blots (A) and quantification (B–F) of RIPA lysates that were probed with the CT20 APP, LC3, Becn1, Pik3c3, and Atg5 antibody. An actin antibody was used as a loading control. 7 AD and 10 control cases were used. BECN1 and PIK3C3 levels were significantly reduced in AD cases (B–C). A significant linear correlation exists between BECN1 and PIK3C3 levels (R = 0.86, p<0.0001), consistent with them functioning in a complex (D). While ATG5 levels appear unchanged, LC3-I and LC3-II levels are significantly elevated (E). A slight trend was detected in LC3-II/LC3-I ratio change (F). No significant difference could be detected in the levels of a neuronal marker NSE between the control and AD brains, indicating that the observed changes are not due to gross neuronal loss (G and H). All scattergrams show mean ± SEM. Means were compared by unpaired Student's t test. * p<0.05, ** p<0.01, *** p<0.001
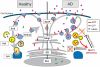
Figure 8. Effects of BECN1 deficiency in AD.
In healthy individuals, APP is transcribed in the endoplasmatic reticulum (ER, grey), modified in the golgi network (Golgi, grey) and then shuttled to the cell surface through the secretory pathway (SecP, grey). The cell takes up APP through endocytosis (End, light blue). From here, APP can either be degraded via autophagy (Aut, yellow) and the lysosomes (Lys, dark blue) or APP can be recycled via the recycling endosomes (R-End, light blue) and enter the cycle again. In AD brains and Becn1 deficient cells BECN1 deficiency impairs both induction of autophagy (through the complex with PIK3C3) and autophagosomal degradation (potentially through a complex with an unknown binding partner). APP containing vesicles (endosomes, autophagosomes, and others) build up inside the cell. APP is increasingly cleaved by secretases and large amounts of APP-CTF and Aβ are being released, causing neurotoxic events. The disruption of autophagosomal degradation includes an increasing accumulation of autophagosomes. This accumulation can further inhibit autophagy and BECN1 expression (red arrow), worsening the reduction in APP turnover and degradation.
Similar articles
- Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.
Salminen A, Kaarniranta K, Kauppinen A, Ojala J, Haapasalo A, Soininen H, Hiltunen M. Salminen A, et al. Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review. - BECN1/Beclin 1 sorts cell-surface APP/amyloid β precursor protein for lysosomal degradation.
Swaminathan G, Zhu W, Plowey ED. Swaminathan G, et al. Autophagy. 2016 Dec;12(12):2404-2419. doi: 10.1080/15548627.2016.1234561. Epub 2016 Oct 7. Autophagy. 2016. PMID: 27715386 Free PMC article. - Divergent roles of BECN1 in LC3 lipidation and autophagosomal function.
He R, Peng J, Yuan P, Xu F, Wei W. He R, et al. Autophagy. 2015;11(5):740-7. doi: 10.1080/15548627.2015.1034404. Autophagy. 2015. PMID: 25955014 Free PMC article. - The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice.
Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, Wyss-Coray T. Pickford F, et al. J Clin Invest. 2008 Jun;118(6):2190-9. doi: 10.1172/JCI33585. J Clin Invest. 2008. PMID: 18497889 Free PMC article. - Acetylation in the regulation of autophagy.
Xu Y, Wan W. Xu Y, et al. Autophagy. 2023 Feb;19(2):379-387. doi: 10.1080/15548627.2022.2062112. Epub 2022 Apr 18. Autophagy. 2023. PMID: 35435793 Free PMC article. Review.
Cited by
- Enhancing the retrograde axonal transport by curcumin promotes autophagic flux in N2a/APP695swe cells.
Liang J, Zhou F, Xiong X, Zhang X, Li S, Li X, Gao M, Li Y. Liang J, et al. Aging (Albany NY). 2019 Sep 6;11(17):7036-7050. doi: 10.18632/aging.102235. Epub 2019 Sep 6. Aging (Albany NY). 2019. PMID: 31488728 Free PMC article. - Acylated Ghrelin as a Multi-Targeted Therapy for Alzheimer's and Parkinson's Disease.
Reich N, Hölscher C. Reich N, et al. Front Neurosci. 2020 Dec 14;14:614828. doi: 10.3389/fnins.2020.614828. eCollection 2020. Front Neurosci. 2020. PMID: 33381011 Free PMC article. Review. - Immunity and inflammation in neurodegenerative diseases.
Cappellano G, Carecchio M, Fleetwood T, Magistrelli L, Cantello R, Dianzani U, Comi C. Cappellano G, et al. Am J Neurodegener Dis. 2013 Jun 21;2(2):89-107. Print 2013. Am J Neurodegener Dis. 2013. PMID: 23844334 Free PMC article. - Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study.
Cribbs DH, Berchtold NC, Perreau V, Coleman PD, Rogers J, Tenner AJ, Cotman CW. Cribbs DH, et al. J Neuroinflammation. 2012 Jul 23;9:179. doi: 10.1186/1742-2094-9-179. J Neuroinflammation. 2012. PMID: 22824372 Free PMC article. - Regulation of neuronal autophagy and the implications in neurodegenerative diseases.
Cai Q, Ganesan D. Cai Q, et al. Neurobiol Dis. 2022 Jan;162:105582. doi: 10.1016/j.nbd.2021.105582. Epub 2021 Dec 7. Neurobiol Dis. 2022. PMID: 34890791 Free PMC article. Review.
References
- Haass C, Selkoe D. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007:101–112. - PubMed
- Laferla F, Green K, Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat Rev Neurosci. 2007:499–509. - PubMed
- Ballatore C, Lee VM, Trojanowski J. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci. 2007:663–672. - PubMed
- Golde TE, Dickson D, Hutton M. Filling the gaps in the abeta cascade hypothesis of Alzheimer's disease. Curr Alzheimer Res. 2006;3:421–430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG10435/AG/NIA NIH HHS/United States
- AG030144/AG/NIA NIH HHS/United States
- R01 AG018440/AG/NIA NIH HHS/United States
- AG02270/AG/NIA NIH HHS/United States
- AG22074/AG/NIA NIH HHS/United States
- AG5131/AG/NIA NIH HHS/United States
- P50 AG005131/AG/NIA NIH HHS/United States
- R01 AG030144/AG/NIA NIH HHS/United States
- R37 AG018440/AG/NIA NIH HHS/United States
- P01 AG010435/AG/NIA NIH HHS/United States
- AG18440/AG/NIA NIH HHS/United States
- P01 AG022074/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous