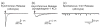Cell biology of Ca2+-triggered exocytosis - PubMed (original) (raw)
Review
Cell biology of Ca2+-triggered exocytosis
Zhiping P Pang et al. Curr Opin Cell Biol. 2010 Aug.
Abstract
Ca(2+) triggers many forms of exocytosis in different types of eukaryotic cells, for example synaptic vesicle exocytosis in neurons, granule exocytosis in mast cells, and hormone exocytosis in endocrine cells. Work over the past two decades has shown that synaptotagmins function as the primary Ca(2+)-sensors for most of these forms of exocytosis, and that synaptotagmins act via Ca(2+)-dependent interactions with both the fusing phospholipid membranes and the membrane fusion machinery. However, some forms of Ca(2+)-induced exocytosis may utilize other, as yet unidentified Ca(2+)-sensors, for example, slow synaptic exocytosis mediating asynchronous neurotransmitter release. In the following overview, we will discuss the synaptotagmin-based mechanism of Ca(2+)-triggered exocytosis in neurons and neuroendocrine cells, and its potential extension to other types of Ca(2+)-stimulated exocytosis for which no synaptotagmin Ca(2+)-sensor has been identified.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
Figure 1. Synaptic and endocrine Ca2+-triggered exocytosis
At a synapse (left), neurotransmitters are packaged into small synaptic vesicles, which are docked at the active zone adjacent to voltage-dependent Ca2+-channels. A presynatpic action potential (insert) gates Ca2+-influx into the terminal, thereby triggering vesicle exocytosis. The released transmitters produce a postsynaptic current (insert) which can be recorded by whole-cell patch clamping. In endocrine cells (right), hormones are packaged into LDCVs, which are generally not docked. Upon sustained increases in cytosolic Ca2+, as obtained during stimulation or Ca2+-uncaging (insert), exocytosis is triggered with a significantly slower time course than at a synapse, as measured by amperometry or capacintance (Cm; insert). Note that Ca2+-channel and release sites are not tightly coupled in endocrine cells. ER, endoplasmic reticulum; M, mitochondrion. Traces are shown purely for demonstration purposes.
Figure 2. Synaptic vesicle exocytosis detected by whole-cell patch clamp recordings
Images depict representative traces of postsynaptic currents illustrating the three different forms of synaptic exocytosis: evoked synchronous release from wild-type synapses (a), evoked asynchronous release from Syt1-deficient synapses (b) and spontaneous mini release (c). Note that asynchronous release also can be recorded in some wild-type neurons upon high-frequency stimulation.
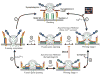
Figure 3. Model of the molecular steps mediated synaptic vesicle exocytosis (modified from [38])
Synaptic vesicles are docked at the active zone of a presynaptic terminal with unassembled SNARE complexes (top)m and are then primed for release by partial SNARE-complex assembly that is catalyzed by Munc18, Munc13, and RIM (step 1). At least in inhibitory synapses, this priming process might be further modulated by ELKS2. The primed vesicles form the substrate for two main pathways of Ca2+-triggered neurotransmitter release: asynchronous release (steps 2 and 3), in which full assembly of SNARE complexes leads to fusion-pore opening followed by complete fusion (step 3); and synchronous release (steps 4,5 and 6), in which “superpriming” by binding of complexins to assembled SNARE complexes (step 4) activates and freezes SNARE complexes in a metastable state (referred to as priming stage II). This stage is then substrate for fast Ca2+-triggering of release when Ca2+-binding to Syt1 induces its binding to phospholipids and to SNARE complexes, with the latter reaction displacing complexin and resulting in fusion-pore opening (step 5) and full fusion (step 6). Both the synchronous and the asynchronous release pathway can mediate spontaneous ‘mini’ release, depending on the local Ca2+-microdomain. Synaptotagmin and complexin clamp (block, in red) the unidentified slow Ca2+-sensor which mediates the asynchronous release; this clamping is relieved when Ca2+ binds to Syt1, allowing competition between Syt1 and the asynchronous Ca2+-sensor during high-frequency stimulation [12,44].
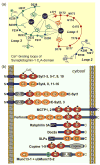
Figure 4. Structures of multiple C2-domain proteins
A. Schematic diagram of the top Ca2+-binding loops of the Syt1 C2A-domain (modified from [41]). The C2A-domain has three top loops, of which loops 1 and 3 form three Ca2+-binding sites. Five aspartate residues, one serine residue and two backbone carbonyl groups coordinate the three bound Ca2+ ions. Residues are shown in single-letter aminio acid code and are identified by residue numbers corresponding to mouse Syt1. B. Domain structures of proteins with multiple C2-domains that contain or lack a transmembrane domain. The mammalian genome encodes four classes of multiple C2-domain proteins containing transmembrane regions: synaptotagmins (Syt1-16; note that ‘Syt17’ does not contain a transmembrane region, but is membrane-anchored via palmitoylated cysteine residues), extended synaptotagmins (E-Syt1-3), multiple C2-domain and transmembrane proteins (MCTP1-2) and ferlins (including otoferlin, dysferlin, myoferlin and 2 other uncharacterized ferlin like proteins). Examples of soluble C2-domain containing proteins are also included. C2-domains with canonical Ca2+-binding consensus sequences are labeled as Ca2+-binding C2-domains, although Ca2+-binding has not yet been tested to all of these domains. C2-domains were assigned based on the conserved domain database of the NCBI; some proteins, especially ferlins, may have additional unpredicted C2-domains that do not precisely fit the consensus sequence, as well as alternative transcripts with fewer C2-domains.
Similar articles
- Synaptotagmin-7 Is Essential for Ca2+-Triggered Delayed Asynchronous Release But Not for Ca2+-Dependent Vesicle Priming in Retinal Ribbon Synapses.
Luo F, Bacaj T, Südhof TC. Luo F, et al. J Neurosci. 2015 Aug 5;35(31):11024-33. doi: 10.1523/JNEUROSCI.0759-15.2015. J Neurosci. 2015. PMID: 26245964 Free PMC article. - Allosteric stabilization of calcium and phosphoinositide dual binding engages several synaptotagmins in fast exocytosis.
Kobbersmed JRL, Berns MMM, Ditlevsen S, Sørensen JB, Walter AM. Kobbersmed JRL, et al. Elife. 2022 Aug 5;11:e74810. doi: 10.7554/eLife.74810. Elife. 2022. PMID: 35929728 Free PMC article. - Synaptotagmin VII as a plasma membrane Ca(2+) sensor in exocytosis.
Sugita S, Han W, Butz S, Liu X, Fernández-Chacón R, Lao Y, Südhof TC. Sugita S, et al. Neuron. 2001 May;30(2):459-73. doi: 10.1016/s0896-6273(01)00290-2. Neuron. 2001. PMID: 11395007 - How does synaptotagmin trigger neurotransmitter release?
Chapman ER. Chapman ER. Annu Rev Biochem. 2008;77:615-41. doi: 10.1146/annurev.biochem.77.062005.101135. Annu Rev Biochem. 2008. PMID: 18275379 Review. - Calcium control of neurotransmitter release.
Südhof TC. Südhof TC. Cold Spring Harb Perspect Biol. 2012 Jan 1;4(1):a011353. doi: 10.1101/cshperspect.a011353. Cold Spring Harb Perspect Biol. 2012. PMID: 22068972 Free PMC article. Review.
Cited by
- Stronger stimulus triggers synaptic transmission faster through earlier started action potential.
Zhang Z, Huang R. Zhang Z, et al. Cell Commun Signal. 2024 Jan 12;22(1):34. doi: 10.1186/s12964-024-01483-3. Cell Commun Signal. 2024. PMID: 38217015 Free PMC article. - Calcium Contributes to Polarized Targeting of HIV Assembly Machinery by Regulating Complex Stability.
Kishor C, Spillings BL, Luhur J, Lutomski CA, Lin CH, McKinstry WJ, Day CJ, Jennings MP, Jarrold MF, Mak J. Kishor C, et al. JACS Au. 2022 Feb 1;2(2):522-530. doi: 10.1021/jacsau.1c00563. eCollection 2022 Feb 28. JACS Au. 2022. PMID: 35253001 Free PMC article. - Synaptic vesicle-like lipidome of human cytomegalovirus virions reveals a role for SNARE machinery in virion egress.
Liu ST, Sharon-Friling R, Ivanova P, Milne SB, Myers DS, Rabinowitz JD, Brown HA, Shenk T. Liu ST, et al. Proc Natl Acad Sci U S A. 2011 Aug 2;108(31):12869-74. doi: 10.1073/pnas.1109796108. Epub 2011 Jul 18. Proc Natl Acad Sci U S A. 2011. PMID: 21768361 Free PMC article. - UNC-13L, UNC-13S, and Tomosyn form a protein code for fast and slow neurotransmitter release in Caenorhabditis elegans.
Hu Z, Tong XJ, Kaplan JM. Hu Z, et al. Elife. 2013 Aug 13;2:e00967. doi: 10.7554/eLife.00967. Elife. 2013. PMID: 23951547 Free PMC article. - A network of interactions enables CCM3 and STK24 to coordinate UNC13D-driven vesicle exocytosis in neutrophils.
Zhang Y, Tang W, Zhang H, Niu X, Xu Y, Zhang J, Gao K, Pan W, Boggon TJ, Toomre D, Min W, Wu D. Zhang Y, et al. Dev Cell. 2013 Oct 28;27(2):215-226. doi: 10.1016/j.devcel.2013.09.021. Dev Cell. 2013. PMID: 24176643 Free PMC article.
References
- Südhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. - PubMed
- Bean AJ, Zhang X, Hokfelt T. Peptide secretion: what do we know? FASEB J. 1994;8:630–638. - PubMed
- Lindau M, Gomperts BD. Techniques and concepts in exocytosis: focus on mast cells. Biochim Biophys Acta. 1991;1071:429–471. - PubMed
- Randriamampita C, Trautmann A. Ca2+ signals and T lymphocytes; “New mechanisms and functions in Ca2+ signalling”. Biol Cell. 2004;96:69–78. - PubMed
- Katz B, Miledi R. Ionic requirements of synaptic transmitter release. Nature. 1967;215:651. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous