Estimating the global clinical burden of Plasmodium falciparum malaria in 2007 - PubMed (original) (raw)
Estimating the global clinical burden of Plasmodium falciparum malaria in 2007
Simon I Hay et al. PLoS Med. 2010.
Abstract
Background: The epidemiology of malaria makes surveillance-based methods of estimating its disease burden problematic. Cartographic approaches have provided alternative malaria burden estimates, but there remains widespread misunderstanding about their derivation and fidelity. The aims of this study are to present a new cartographic technique and its application for deriving global clinical burden estimates of Plasmodium falciparum malaria for 2007, and to compare these estimates and their likely precision with those derived under existing surveillance-based approaches.
Methods and findings: In seven of the 87 countries endemic for P. falciparum malaria, the health reporting infrastructure was deemed sufficiently rigorous for case reports to be used verbatim. In the remaining countries, the mapped extent of unstable and stable P. falciparum malaria transmission was first determined. Estimates of the plausible incidence range of clinical cases were then calculated within the spatial limits of unstable transmission. A modelled relationship between clinical incidence and prevalence was used, together with new maps of P. falciparum malaria endemicity, to estimate incidence in areas of stable transmission, and geostatistical joint simulation was used to quantify uncertainty in these estimates at national, regional, and global scales. Combining these estimates for all areas of transmission risk resulted in 451 million (95% credible interval 349-552 million) clinical cases of P. falciparum malaria in 2007. Almost all of this burden of morbidity occurred in areas of stable transmission. More than half of all estimated P. falciparum clinical cases and associated uncertainty occurred in India, Nigeria, the Democratic Republic of the Congo (DRC), and Myanmar (Burma), where 1.405 billion people are at risk. Recent surveillance-based methods of burden estimation were then reviewed and discrepancies in national estimates explored. When these cartographically derived national estimates were ranked according to their relative uncertainty and replaced by surveillance-based estimates in the least certain half, 98% of the global clinical burden continued to be estimated by cartographic techniques.
Conclusions and significance: Cartographic approaches to burden estimation provide a globally consistent measure of malaria morbidity of known fidelity, and they represent the only plausible method in those malaria-endemic countries with nonfunctional national surveillance. Unacceptable uncertainty in the clinical burden of malaria in only four countries confounds our ability to evaluate needs and monitor progress toward international targets for malaria control at the global scale. National prevalence surveys in each nation would reduce this uncertainty profoundly. Opportunities for further reducing uncertainty in clinical burden estimates by hybridizing alternative burden estimation procedures are also evaluated.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
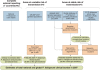
Figure 1. Schematic diagram showing the procedure for burden estimation.
Blue boxes describe input data, orange boxes models and experimental procedures, dashed green rods intermediate output, and solid green rods the final output. The seven countries with reliable national reporting were Belize, Iran, Kyrgyzstan, Panama, Saudi Arabia, South Africa, and Tajikistan. The areas of unstable and stable transmission are defined as having less or more than one case per 10,000 PA, respectively ,.
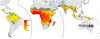
Figure 2. Global limits and endemicity of P. falciparum in 2007.
The land area was defined as no risk (light grey), unstable risk (medium grey areas, where _Pf_API <0.1‰ PA), and stable risk (where _Pf_API >0.1‰ PA) with endemicity (_Pf_PR in the 2- up to 10-year age group, _Pf_PR2–10) displayed as a continuum of yellow to red between 0% and 100%. The dashed lines separate the Americas, Africa+, and the CSE Asia region, respectively, from left to right. The seven countries with thick blue borders have very low P. falciparum burden and reliable national health information systems.
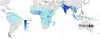
Figure 3. Global human population density in 2007.
Human population density in persons per km2 is displayed on a logarithmic colour scale within the limits of P. falciparum transmission. No malaria risk is shown in light grey.
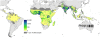
Figure 4. Global clinical burden of P. falciparum in 2007.
Bayesian geostatistical estimates (posterior means) of the number of all-age clinical cases per 5×5 km pixel displayed on a logarithmic colour scale between 0 and 10,000 cases, within the stable limits of P. falciparum transmission. Dark and light grey areas are as described in Figure 2.
Figure 5. Uncertainty in the global clinical burden of P. falciparum in 2007.
Bayesian geostatistical model-based prediction uncertainty (posterior standard deviations) on a logarithmic colour scale between 0 and 20,000 cases, within the stable limits of P. falciparum transmission. No model-based uncertainty metrics were produced for areas of unstable transmission. Dark and light grey areas are as described in Figure 2.
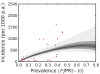
Figure 6. The posterior distribution of the prevalence-incidence relationship (, see Methods) in the Africa+ region.
The relationship is plotted between malaria endemicity (_Pf_PR in the 2- up to 10-year age group, _Pf_PR2–10) and all-age incidence (clinical cases per thousand of the population PA) . Please see reference for a full description of the data, methods, and techniques used to define this relationship. The light grey, medium grey and dark grey regions define the 95%, 50%, and 25% credible intervals, respectively. The solid black line is the median and the data are shown as red dots.
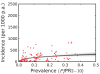
Figure 7. The posterior distribution of the prevalence-incidence relationship (, see Methods) in the combined CSE Asia region and the Americas.
The techniques and colours used are identical to Figure 6.
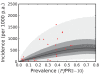
Figure 8. The predictive distribution of the incidence that would actually be observed by weekly surveillance over a two-year period in the Africa+ region.
Please see reference for a full description of the data, methods, and techniques used to define this relationship. The light grey, medium grey, and dark grey regions define the 95%, 50%, and 25% credible intervals, respectively. The solid black line is the median and the data are shown as red dots. Note that the data points were collected using different surveillance intervals over different time periods, and therefore should not be expected to follow the distribution predicted by the model exactly. The observed incidences are included in the figure as a visual aid only.
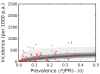
Figure 9. The predictive distribution of the incidence that would actually be observed by weekly surveillance over a two-year period in the combined CSE Asia region and the Americas.
The techniques and colours used are identical to Figure 8.
Figure 10. Pie chart of P. falciparum clinical cases in 2007.
The pie chart shows the fraction of the 451 million cases of total clinical burden in each of the World Health Organization regions (Protocol S2). In the pie the regions are ordered counterclockwise starting at the top, from highest to lowest burden. The plotted area representing the EURO region is too thin to be visible. The thumbnail map shows the country composition of the WHO regions for all 87 P. falciparum endemic countries.
References
- Snow RW, Craig MH, Newton CRJC, Steketee RW. The public health burden of Plasmodium falciparum malaria in Africa: deriving the numbers. 2003. pp. 1–71. Working Paper No. 11, Disease Control Priorities Project Bethesda, Maryland, U.S.A.: Fogarty International Centre, National Institutes of Health.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical