The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson's disease and Parkinson's disease dementia - PubMed (original) (raw)
Review
The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson's disease and Parkinson's disease dementia
Walter J Schulz-Schaeffer. Acta Neuropathol. 2010 Aug.
Abstract
Parkinson's disease (PD) and dementia with Lewy bodies (DLB) are usually associated with loss of dopaminergic neurons. Loss of substantia nigra neurons and presence of Lewy body inclusions in some of the remaining neurons are the hallmark pathology seen in the final stages of the disease. Attempts to correlate Lewy body pathology to either cell death or severity of clinical symptoms, however, have not been successful. While the pathophysiology of the neurodegenerative process can hardly be explained by Lewy bodies, the clinical symptoms do indicate a degenerative process located at the presynapse resulting in a neurotransmitter deficiency. Recently it was shown that 90% or even more of alpha-synuclein aggregates in DLB cases were located at the presynapses in the form of very small deposits. In parallel, dendritic spines are retracted, whereas the presynapses are relatively preserved, suggesting a neurotransmitter deprivation. The same alpha-synuclein pathology can be demonstrated for PD. These findings give rise to the notion that not cell death but rather alpha-synuclein aggregate-related synaptic dysfunction causes the neurodegeneration. This opens new perspectives for understanding PD and DLB. If presynaptic alpha-synuclein aggregation, not neuronal loss, is the key issue of the neurodegenerative process, then PD and DLB may eventually be treatable in the future. The disease may progress via trans-synaptical spread, suggesting that stem cell transplants are of limited use. Future therapies may focus on the regeneration of synapses.
Figures
Fig. 1
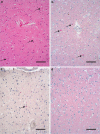
Detection of α-synuclein deposits in DLB with conventional methods. H&E (a) shows 3 Lewy bodies (arrows). Immunohistochemically, Lewy bodies are detectable (b), whereas in the neuropil α-synuclein deposits are not distinguishable from the physiological α-synuclein staining (d) as compared to a control case (mAB 4B12, 1:1,000, abcam). With an antibody against phosphorylated α-synuclein (c), more deposits than just Lewy bodies are detectable (polyAB pSer129, 1:500, LifeSpan BioScience). Bar 100 μm
Fig. 2
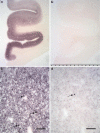
More than 90% of α-synuclein aggregates are located outside of Lewy bodies at synapses in the frontal cortex of DLB. Frontal cortex and cingulate gyrus of a DLB- and control patient as seen using a dissection microscope (a, b). The higher magnification of the PET blot (c) shows the synaptic distribution of aggregates much smaller than Lewy bodies (LB indicated by arrows; mAB 4B12, 1:10,000). Using an antibody against phosphorylated α-synuclein (d), only a fraction of proteinase K-resistant aggregates is detectable (polyAB pSer129, 1:5,000). The detectability of phosphorylated α-synuclein is strongly influenced by the fixation period. Here the tissue was fixated short term using buffered formaldehyde. Bar 100 μm
Fig. 3
Synaptic α-synuclein aggregates are the main synuclein pathology in Parkinson’s disease as seen in DLB. The substantia nigra shows several proteinase K-resistant α-synuclein aggregates besides Lewy bodies (a). In a Parkinson’s disease patient with dementia (b), the frontal cortex shows a lot of tiny α-synuclein aggregates even though no Lewy bodies are detectable (mAB 4B12, 1:10,000). Bar 100 μm
Fig. 4
An almost complete loss of dendritic spines accompanies the presynaptic α-synuclein aggregates. Golgy–Cox–Davenport staining of a neuronal dendrite of a frontal cortex neuron in a DLB patient (a) is compared to a control patient of the same age (b). Bar 50 μm
Similar articles
- Is Cell Death Primary or Secondary in the Pathophysiology of Idiopathic Parkinson's Disease?
Schulz-Schaeffer WJ. Schulz-Schaeffer WJ. Biomolecules. 2015 Jul 16;5(3):1467-79. doi: 10.3390/biom5031467. Biomolecules. 2015. PMID: 26193328 Free PMC article. Review. - Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies.
Kramer ML, Schulz-Schaeffer WJ. Kramer ML, et al. J Neurosci. 2007 Feb 7;27(6):1405-10. doi: 10.1523/JNEUROSCI.4564-06.2007. J Neurosci. 2007. PMID: 17287515 Free PMC article. - [Clinical and pathological study on early diagnosis of Parkinson's disease and dementia with Lewy bodies].
Orimo S. Orimo S. Rinsho Shinkeigaku. 2008 Jan;48(1):11-24. doi: 10.5692/clinicalneurol.48.11. Rinsho Shinkeigaku. 2008. PMID: 18386627 Review. Japanese. - A critical evaluation of current staging of alpha-synuclein pathology in Lewy body disorders.
Jellinger KA. Jellinger KA. Biochim Biophys Acta. 2009 Jul;1792(7):730-40. doi: 10.1016/j.bbadis.2008.07.006. Epub 2008 Aug 5. Biochim Biophys Acta. 2009. PMID: 18718530 Review. - Pathology-associated change in levels and localization of SIDT2 in postmortem brains of Parkinson's disease and dementia with Lewy bodies patients.
Fujiwara Y, Kabuta C, Sano T, Murayama S, Saito Y, Kabuta T. Fujiwara Y, et al. Neurochem Int. 2022 Jan;152:105243. doi: 10.1016/j.neuint.2021.105243. Epub 2021 Nov 18. Neurochem Int. 2022. PMID: 34800582
Cited by
- α-Synuclein-112 Impairs Synaptic Vesicle Recycling Consistent With Its Enhanced Membrane Binding Properties.
Soll LG, Eisen JN, Vargas KJ, Medeiros AT, Hammar KM, Morgan JR. Soll LG, et al. Front Cell Dev Biol. 2020 May 29;8:405. doi: 10.3389/fcell.2020.00405. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32548120 Free PMC article. - Controlled cortical impact results in an extensive loss of dendritic spines that is not mediated by injury-induced amyloid-beta accumulation.
Winston CN, Chellappa D, Wilkins T, Barton DJ, Washington PM, Loane DJ, Zapple DN, Burns MP. Winston CN, et al. J Neurotrauma. 2013 Dec 1;30(23):1966-72. doi: 10.1089/neu.2013.2960. Epub 2013 Oct 12. J Neurotrauma. 2013. PMID: 23879560 Free PMC article. - Synaptic protein alterations in Parkinson's disease.
Pienaar IS, Burn D, Morris C, Dexter D. Pienaar IS, et al. Mol Neurobiol. 2012 Feb;45(1):126-43. doi: 10.1007/s12035-011-8226-9. Epub 2011 Dec 29. Mol Neurobiol. 2012. PMID: 22205299 Review. - Alpha-synuclein: from secretion to dysfunction and death.
Marques O, Outeiro TF. Marques O, et al. Cell Death Dis. 2012 Jul 19;3(7):e350. doi: 10.1038/cddis.2012.94. Cell Death Dis. 2012. PMID: 22825468 Free PMC article. Review. - A First Tetraplex Assay for the Simultaneous Quantification of Total α-Synuclein, Tau, β-Amyloid42 and DJ-1 in Human Cerebrospinal Fluid.
Kruse N, Schlossmacher MG, Schulz-Schaeffer WJ, Vanmechelen E, Vanderstichele H, El-Agnaf OM, Mollenhauer B. Kruse N, et al. PLoS One. 2016 Apr 26;11(4):e0153564. doi: 10.1371/journal.pone.0153564. eCollection 2016. PLoS One. 2016. PMID: 27116005 Free PMC article.
References
- Akopian AN, Wood JN. Peripheral nervous system-specific genes identified by subtractive cDNA cloning. J Biol Chem. 1995;270:21264–21270. - PubMed
- Andreoletti O, Simon S, Lacroux C, et al. PrPSc accumulation in myocytes from sheep incubating natural scrapie. Nat Med. 2004;10:591–593. - PubMed
- Aoki C, Sekino Y, Hanamura K, et al. Drebrin A is a postsynaptic protein that localizes in vivo to the submembranous surface of dendritic sites forming excitatory synapses. J Comp Neurol. 2005;483:383–402. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical