Array comparative hybridisation reveals a high degree of similarity between UK and European clinical isolates of hypervirulent Clostridium difficile - PubMed (original) (raw)
Array comparative hybridisation reveals a high degree of similarity between UK and European clinical isolates of hypervirulent Clostridium difficile
Gemma L Marsden et al. BMC Genomics. 2010.
Abstract
Background: Clostridium difficile is a Gram-positive, anaerobic, spore-forming bacterium that is responsible for C. difficile associated disease in humans and is currently the most common cause of nosocomial diarrhoea in the western world. This current status has been linked to the emergence of a highly virulent PCR-ribotype 027 strain. The aim of this work was to identify regions of sequence divergence that may be used as genetic markers of hypervirulent PCR-ribotype 027 strains and markers of the sequenced strain, CD630 by array comparative hybridisation.
Results: In this study, we examined 94 clinical strains of the most common PCR-ribotypes isolated in mainland Europe and the UK by array comparative genomic hybridisation. Our array was comprehensive with 40,097 oligonucleotides covering the C. difficile 630 genome and revealed a core genome for all the strains of 32%. The array also covered genes unique to two PCR-ribotype 027 strains, relative to C. difficile 630 which were represented by 681 probes. All of these genes were also found in the commonly occuring PCR-ribotypes 001 and 106, and the emerging hypervirulent PCR-ribotype 078 strains, indicating that these are markers for all highly virulent strains.
Conclusions: We have fulfilled the aims of this study by identifying markers for CD630 and markers associated with hypervirulence, albeit genes that are not just indicative of PCR-ribotype 027 strains. We have also extended this study and have defined a more stringent core gene set compared to those previously published due to the comprehensive array coverage. Further to this we have defined a list of genes absent from non-toxinogenic strains and defined the nature of the specific toxin deletion in the strain CD37.
Figures
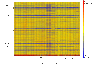
Figure 1
Comparative genomic analysis of 94 strains of clinical strains of C. difficile. The probes were arranged by their corresponding C. difficile 630 gene, with CD0001 at the top and CD3680 at the bottom, followed by CDS from the plasmid pCD630 (CDP01 to CDP11) and finally probes representing the genes unique to ribotype 027. Each column represents an isolate, and each row corresponds to a probe. The status of each probe is indicated by color as follows: red, present/conserved in the test strain; blue, absent in the test strain and yellow present in both the test and control strains. The strains are grouped by PCR-ribotype and this is indicated below. The writing on the left indicates regions of divergence from CD630 in all of the strains tested.
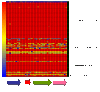
Figure 2
Schematic diagram produced by GeneSpring showing all the oligonucleotides representing the second agr locus against all the clinical strains. The gene context of region is detailed below the diagram but this is not to scale. Each row represents an isolate, and each column corresponds to a probe. Strain PCR-ribotypes are indicated on the right. The status of each probe is indicated by color as follows: red, present/conserved in the test strain; blue, absent in the test strain and yellow in this case were the region is absent in CD630 indicates divergence in these genes.
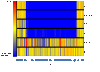
Figure 3
Schematic diagram produced by GeneSpring showing all the oligonucleotides representing the PaLoc against CD630, R20291 and the 4 non-toxigenic strains tested. This includes probes that will not hybridise to either of our control strains, CD630 and R20291. The gene context of the region is detailed below the diagram but this is not to scale. Each row represents an isolate, and each column corresponds to a probe. The strain ID is indicated on the right. The status of each probe is indicated by color as follows: red, present/conserved in the test strain; blue, absent in the test strain and yellow present in both the test and control strains.
Similar articles
- Comparative phylogenomics of Clostridium difficile reveals clade specificity and microevolution of hypervirulent strains.
Stabler RA, Gerding DN, Songer JG, Drudy D, Brazier JS, Trinh HT, Witney AA, Hinds J, Wren BW. Stabler RA, et al. J Bacteriol. 2006 Oct;188(20):7297-305. doi: 10.1128/JB.00664-06. J Bacteriol. 2006. PMID: 17015669 Free PMC article. - Sequence similarity of Clostridium difficile strains by analysis of conserved genes and genome content is reflected by their ribotype affiliation.
Kurka H, Ehrenreich A, Ludwig W, Monot M, Rupnik M, Barbut F, Indra A, Dupuy B, Liebl W. Kurka H, et al. PLoS One. 2014 Jan 23;9(1):e86535. doi: 10.1371/journal.pone.0086535. eCollection 2014. PLoS One. 2014. PMID: 24482682 Free PMC article. - Comparative genome and phenotypic analysis of Clostridium difficile 027 strains provides insight into the evolution of a hypervirulent bacterium.
Stabler RA, He M, Dawson L, Martin M, Valiente E, Corton C, Lawley TD, Sebaihia M, Quail MA, Rose G, Gerding DN, Gibert M, Popoff MR, Parkhill J, Dougan G, Wren BW. Stabler RA, et al. Genome Biol. 2009;10(9):R102. doi: 10.1186/gb-2009-10-9-r102. Epub 2009 Sep 25. Genome Biol. 2009. PMID: 19781061 Free PMC article. - The Clostridium difficile PCR ribotype 027 lineage: a pathogen on the move.
Valiente E, Cairns MD, Wren BW. Valiente E, et al. Clin Microbiol Infect. 2014 May;20(5):396-404. doi: 10.1111/1469-0691.12619. Epub 2014 Apr 28. Clin Microbiol Infect. 2014. PMID: 24621128 Review. - The emergence of 'hypervirulence' in Clostridium difficile.
Cartman ST, Heap JT, Kuehne SA, Cockayne A, Minton NP. Cartman ST, et al. Int J Med Microbiol. 2010 Aug;300(6):387-95. doi: 10.1016/j.ijmm.2010.04.008. Epub 2010 May 23. Int J Med Microbiol. 2010. PMID: 20547099 Review.
Cited by
- Genetic diversity and epidemiology of accessory gene regulator loci in Clostridioides difficile.
Okada Y, Okugawa S, Ikeda M, Kobayashi T, Saito R, Higurashi Y, Moriya K. Okada Y, et al. Access Microbiol. 2020 May 14;2(7):acmi000134. doi: 10.1099/acmi.0.000134. eCollection 2020. Access Microbiol. 2020. PMID: 32974597 Free PMC article. - Clostridioides difficile ribotype 106: A systematic review of the antimicrobial susceptibility, genetics, and clinical outcomes of this common worldwide strain.
Carlson TJ, Blasingame D, Gonzales-Luna AJ, Alnezary F, Garey KW. Carlson TJ, et al. Anaerobe. 2020 Apr;62:102142. doi: 10.1016/j.anaerobe.2019.102142. Epub 2019 Dec 19. Anaerobe. 2020. PMID: 32007682 Free PMC article. - The Regulatory Networks That Control Clostridium difficile Toxin Synthesis.
Martin-Verstraete I, Peltier J, Dupuy B. Martin-Verstraete I, et al. Toxins (Basel). 2016 May 14;8(5):153. doi: 10.3390/toxins8050153. Toxins (Basel). 2016. PMID: 27187475 Free PMC article. Review. - The LexA regulated genes of the Clostridium difficile.
Walter BM, Rupnik M, Hodnik V, Anderluh G, Dupuy B, Paulič N, Žgur-Bertok D, Butala M. Walter BM, et al. BMC Microbiol. 2014 Apr 8;14:88. doi: 10.1186/1471-2180-14-88. BMC Microbiol. 2014. PMID: 24713082 Free PMC article. - The agr locus regulates virulence and colonization genes in Clostridium difficile 027.
Martin MJ, Clare S, Goulding D, Faulds-Pain A, Barquist L, Browne HP, Pettit L, Dougan G, Lawley TD, Wren BW. Martin MJ, et al. J Bacteriol. 2013 Aug;195(16):3672-81. doi: 10.1128/JB.00473-13. Epub 2013 Jun 14. J Bacteriol. 2013. PMID: 23772065 Free PMC article.
References
- Elixhauser A, Jhung M. HCUP statistical brief No. 50. US Agency for Healthcare Research and Quality, Rockville, MD; 2008. Clostridium difficile-associated disease in US hospitals, 1993-2005. - PubMed
- Barbut F, Gariazzo B, Bonne L, Lalande V, Burghoffer B, Luiuz R, Petit JC. Clinical features of Clostridium difficile-associated infections and molecular characterization of strains: results of a retrospective study, 2000-2004. Infect Control Hosp Epidemiol. 2007;28:131–139. doi: 10.1086/511794. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources