Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns - PubMed (original) (raw)
Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns
Maria G Dominguez-Bello et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Upon delivery, the neonate is exposed for the first time to a wide array of microbes from a variety of sources, including maternal bacteria. Although prior studies have suggested that delivery mode shapes the microbiota's establishment and, subsequently, its role in child health, most researchers have focused on specific bacterial taxa or on a single body habitat, the gut. Thus, the initiation stage of human microbiome development remains obscure. The goal of the present study was to obtain a community-wide perspective on the influence of delivery mode and body habitat on the neonate's first microbiota. We used multiplexed 16S rRNA gene pyrosequencing to characterize bacterial communities from mothers and their newborn babies, four born vaginally and six born via Cesarean section. Mothers' skin, oral mucosa, and vagina were sampled 1 h before delivery, and neonates' skin, oral mucosa, and nasopharyngeal aspirate were sampled <5 min, and meconium <24 h, after delivery. We found that in direct contrast to the highly differentiated communities of their mothers, neonates harbored bacterial communities that were undifferentiated across multiple body habitats, regardless of delivery mode. Our results also show that vaginally delivered infants acquired bacterial communities resembling their own mother's vaginal microbiota, dominated by Lactobacillus, Prevotella, or Sneathia spp., and C-section infants harbored bacterial communities similar to those found on the skin surface, dominated by Staphylococcus, Corynebacterium, and Propionibacterium spp. These findings establish an important baseline for studies tracking the human microbiome's successional development in different body habitats following different delivery modes, and their associated effects on infant health.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
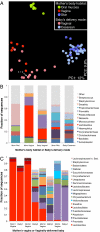
Bacterial 16S rRNA gene surveys reveal that the first microbiotas of human newborns are primarily structured by delivery mode. (A) Communities clustered using principal coordinates analysis of the unweighted UniFrac distance matrix. PC1 and PC2 are plotted on _x_- and _y_-axes. Each point corresponds to a community colored according to the mother's body habitat or the newborn's delivery mode. All newborn body habitats are shown. The percentage of variation explained by the plotted principal coordinates is indicated on the axes. The white arrow indicates a pair of superimposed points. Vaginal samples were not obtained from two of the mothers who delivered by C-section. (B) Average relative abundances of the dominant taxa found in this study in aggregated samples. (C) Relative abundances of the 20 most abundant taxa in mothers’ vaginal communities and in the babies they delivered vaginally. Sequences were classified to highest taxonomic level to which they could be confidently assigned.
Fig. 2.
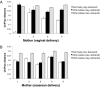
The effect of delivery mode on the direct transmission of bacteria from mother to newborn. Average (±SEM) weighted UniFrac distance for pairwise comparisons between (A) vaginal microbiota of mothers who delivered vaginally, or (B) the skin microbiota of mothers who delivered via C-section and the microbiotas of the newborn babies. The maternal microbiota (vagina or skin) was compared with the microbiota of her own baby, or babies of the same or different delivery mode. Baby samples were from the skin (arms and forehead), oral mucosa, nasopharyngeal aspirate, and meconium. The mothers’ skin samples were from both arms.
Comment in
- Additional maternal and nonmaternal factors contribute to microbiota shaping in newborns.
Putignani L, Carsetti R, Signore F, Manco M. Putignani L, et al. Proc Natl Acad Sci U S A. 2010 Oct 19;107(42):E159; author reply E160. doi: 10.1073/pnas.1010526107. Epub 2010 Sep 27. Proc Natl Acad Sci U S A. 2010. PMID: 20876088 Free PMC article. No abstract available.
Similar articles
- Impact of maternal obesity and mode of delivery on the newborn skin and oral microbiomes.
Seifert A, Ingram K, Eko EN, Nunziato J, Ahrens M, Howell BR. Seifert A, et al. J Med Microbiol. 2025 Apr;74(4). doi: 10.1099/jmm.0.002000. J Med Microbiol. 2025. PMID: 40208663 - Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer.
Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, Cox LM, Amir A, Gonzalez A, Bokulich NA, Song SJ, Hoashi M, Rivera-Vinas JI, Mendez K, Knight R, Clemente JC. Dominguez-Bello MG, et al. Nat Med. 2016 Mar;22(3):250-3. doi: 10.1038/nm.4039. Epub 2016 Feb 1. Nat Med. 2016. PMID: 26828196 Free PMC article. - Delivery mode, birth order, and sex impact neonatal microbial colonization.
Kennedy KM, Plagemann A, Sommer J, Hofmann M, Henrich W, Surette MG, Braun T, Sloboda DM. Kennedy KM, et al. Gut Microbes. 2025 Dec;17(1):2491667. doi: 10.1080/19490976.2025.2491667. Epub 2025 Apr 19. Gut Microbes. 2025. PMID: 40251947 Free PMC article. - Gut Microbiota Composition in Healthy Japanese Infants and Young Adults Born by C-Section.
Nagpal R, Yamashiro Y. Nagpal R, et al. Ann Nutr Metab. 2018;73 Suppl 3:4-11. doi: 10.1159/000490841. Epub 2018 Jul 24. Ann Nutr Metab. 2018. PMID: 30041174 Review. - Impact of vaginal seeding on the gut microbiome of infants born via cesarean section: A systematic review.
Wang X, Cui H, Li N, Liu B, Zhang X, Yang J, Zheng JS, Qiao C, Liu HX, Hu J, Wen D. Wang X, et al. J Infect. 2024 Dec;89(6):106348. doi: 10.1016/j.jinf.2024.106348. Epub 2024 Nov 12. J Infect. 2024. PMID: 39537035 Review.
Cited by
- The human microbiome as a reservoir of antimicrobial resistance.
Penders J, Stobberingh EE, Savelkoul PH, Wolffs PF. Penders J, et al. Front Microbiol. 2013 Apr 17;4:87. doi: 10.3389/fmicb.2013.00087. eCollection 2013. Front Microbiol. 2013. PMID: 23616784 Free PMC article. - Skin-to-Skin Care and the Development of the Preterm Infant Oral Microbiome.
Hendricks-Muñoz KD, Xu J, Parikh HI, Xu P, Fettweis JM, Kim Y, Louie M, Buck GA, Thacker LR, Sheth NU. Hendricks-Muñoz KD, et al. Am J Perinatol. 2015 Nov;32(13):1205-16. doi: 10.1055/s-0035-1552941. Epub 2015 May 22. Am J Perinatol. 2015. PMID: 26007311 Free PMC article. - Multi-omic analyses identify mucosa bacteria and fecal metabolites associated with weight loss after fecal microbiota transplantation.
Zhang F, Zuo T, Wan Y, Xu Z, Cheung C, Li AY, Zhu W, Tang W, Chan PKS, Chan FKL, Ng SC. Zhang F, et al. Innovation (Camb). 2022 Aug 17;3(5):100304. doi: 10.1016/j.xinn.2022.100304. eCollection 2022 Sep 13. Innovation (Camb). 2022. PMID: 36091491 Free PMC article. - Visceral pain and gastrointestinal microbiome.
Chichlowski M, Rudolph C. Chichlowski M, et al. J Neurogastroenterol Motil. 2015 Mar 30;21(2):172-81. doi: 10.5056/jnm15025. J Neurogastroenterol Motil. 2015. PMID: 25829337 Free PMC article. - Microbiota Composition of Breast Milk from Women of Different Ethnicity from the Manawatu-Wanganui Region of New Zealand.
Butts CA, Paturi G, Blatchford P, Bentley-Hewitt KL, Hedderley DI, Martell S, Dinnan H, Eady SL, Wallace AJ, Glyn-Jones S, Wiens F, Stahl B, Gopal P. Butts CA, et al. Nutrients. 2020 Jun 11;12(6):1756. doi: 10.3390/nu12061756. Nutrients. 2020. PMID: 32545413 Free PMC article.
References
- Zhou X, et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J. 2007;1:121–133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous