Lymphotoxin-alpha contributes to lymphangiogenesis - PubMed (original) (raw)
Lymphotoxin-alpha contributes to lymphangiogenesis
Rawad H Mounzer et al. Blood. 2010.
Abstract
Lymphotoxin-α (LTα), lymphotoxin-β (LTβ), and tumor necrosis factor-α (TNFα) are inflammatory mediators that play crucial roles in lymphoid organ development. We demonstrate here that LTα also contributes to the function of lymphatic vessels and to lymphangiogenesis during inflammation. LTα(-/-) mice exhibited reduced lymph flow velocities and increased interstitial fluid pressure. Airways of LTβ(-/-) mice infected with Mycoplasma pulmonis had significantly more lymphangiogenesis than wild type (WT) or LTα(-/-) mice, as did the skin draining immunization sites of LTβ(-/-) mice. Macrophages, B cells, and T cells, known sources of LT and TNFα, were apparent in the skin surrounding the immunization sites as were LTα, LTβ, and TNFα mRNAs. Ectopic expression of LTα led to the development of LYVE-1 and Prox1-positive lymphatic vessels within tertiary lymphoid organs (TLOs). Quantification of pancreatic lymphatic vessel density in RIPLTαLTβ(-/-) and WT mice revealed that LTα was sufficient for inducing lymphangiogenesis and that LTβ was not required for this process. Kidneys of inducible LTα transgenic mice developed lymphatic vessels before the appearance of obvious TLOs. These data indicate that LTα plays a significant role in lymphatic vessel function and in inflammation-associated lymphangiogenesis.
Figures
Figure 1
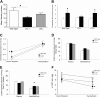
Analysis of lymph flow velocity and physiology. (A) Quantitative analysis using residence time distribution theory reveals a significant difference in the lymph flow velocity of fluorescent dextran in tail lymphatics between LTα−/− and wild type (WT) mice, but not between LTβ−/− and WT mice (a minimum of n = 10 mice per group; *P < .05). (B) Extracellular fluid volume (EFV) in paw skin measured during steady state control situation ( ) and after overhydration (
) and after overhydration ( ). In contrast to LTα−/− mice (n = 8 in control [c] and overhydration [o]), both LTβ−/− (n = 8 [c] and n = 9 [o]) and WT (n = 6 [c] and n = 4 [o]) mice show a significant increase in EFV after overhydration (*P < .05). (C) LTα−/− mice (n = 16) have a significantly higher interstitial fluid pressure (Pif) than both LTβ−/− (n = 17) and WT mice (n = 10) during steady state control situation (*P < .05). All 3 groups, however, demonstrate a significant increase in Pif after overhydration (#P < .05). (D) Colloid osmotic pressure (COP) in plasma and interstitial fluid in the steady state control situation. LTα−/− mice show comparable COP in the interstitial fluid to WT and LTβ−/− mice. (E) After overhydration, the LTα−/− mice (n = 6) show a significantly higher COP in the interstitial fluid in comparison to WT mice (n = 7; *P < .05). (F) Colloid osmotic pressure gradient (ΔCOP) across the capillaries in the steady state control situation and after overhydration. All 3 groups show a nonsignificant decrease in ΔCOP after overhydration. Overhydrated LTα−/− (n = 6) mice have a significantly lower ΔCOP compared with WT (n = 7) mice (*P < .05).
). In contrast to LTα−/− mice (n = 8 in control [c] and overhydration [o]), both LTβ−/− (n = 8 [c] and n = 9 [o]) and WT (n = 6 [c] and n = 4 [o]) mice show a significant increase in EFV after overhydration (*P < .05). (C) LTα−/− mice (n = 16) have a significantly higher interstitial fluid pressure (Pif) than both LTβ−/− (n = 17) and WT mice (n = 10) during steady state control situation (*P < .05). All 3 groups, however, demonstrate a significant increase in Pif after overhydration (#P < .05). (D) Colloid osmotic pressure (COP) in plasma and interstitial fluid in the steady state control situation. LTα−/− mice show comparable COP in the interstitial fluid to WT and LTβ−/− mice. (E) After overhydration, the LTα−/− mice (n = 6) show a significantly higher COP in the interstitial fluid in comparison to WT mice (n = 7; *P < .05). (F) Colloid osmotic pressure gradient (ΔCOP) across the capillaries in the steady state control situation and after overhydration. All 3 groups show a nonsignificant decrease in ΔCOP after overhydration. Overhydrated LTα−/− (n = 6) mice have a significantly lower ΔCOP compared with WT (n = 7) mice (*P < .05).
Figure 2
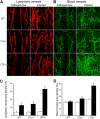
Increased lymphangiogenesis in LTβ−/− mouse tracheas after infection with Mycoplasma pulmonis. (A) LYVE-1+ lymphatic vessels (red) and (B) CD31+ blood vessels (green) increase in the tracheas of mice infected with M pulmonis. Scale bar is 200 μm (Zeiss 510 Confocal Microscope). (C) Quantification reveals a significant increase in lymphatic vessel density in the tracheas of LTβ−/− mice compared with both LTα−/− and WT mice (*P < .05). (D) A similar increase was noted in blood vessel density.
Figure 3
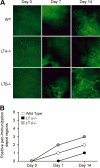
Increased lymphangiogenesis in LTβ−/− mouse skin after induction of inflammation. (A) Fluorescence microscopy of the site of immunization with ovalbumin and CFA after injection of FITC-conjugated nanoparticles reveals more prominent and extensive lymphatic vessel networks near immunization sites in LTβ−/− mice compared with LTα−/− and WT mice. Pale green at day 0 represents autofluorescence. Black blood vessels are apparent and even more obvious after immunization. Lymphatic vessels are bright green (6.5× objective). (B) More peri-immunization depot sites showed uptake of the nanoparticles by lymphatics in LTβ−/− than WT and LTα−/− mice (n = 3 per group with total of 6 depot regions per group).
Figure 4
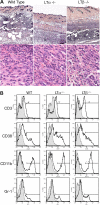
Massive leukocytic infiltrates at immunization sites in the skin. (A) Hematoxylin and eosin staining of the skin at the site of immunization with ovalbumin and CFA on day 7 reveals cellular infiltration of the skin. (original magnifications 5× or 100× oil objective; Zeiss Axioscope). (B) FACS analysis of cells isolated from skin site of immunization reveals consistently increased proportions of macrophages and granulocytes in skin from LTβ−/− mice (representative of 4 experiments at days 4 and 7; n = 4 mice per group).
Figure 5
Lymphatic vessels associated with tertiary lymphoid organs in the pancreas induced by ectopic expression of LTα. (A) H&E, DAPI, and Prox1 staining of 1-year-old RIPLTα pancreas (40× objective; Zeiss Axioscope). (B) Merge of peri-islet bright field staining for hematoxylin and dark field for LYVE-1 positive lymphatic vessels (red) in the pancreas. More lymphatic vessels are associated with TLOs around the islets of RIPLTα and RIPLTαLTβ−/− mice than WT mice (n = 3 per group; 20× objective; Zeiss Axioscope). (C) Morphometric quantification reveals a significant increase in peri-islet LYVE-1 positive vessel area in the pancreata of RIPLTα and RIPLTαLTβ−/− mice compared with WT mice (*P < .05). No significant difference in LYVE-1 positive vessel area was found between RIPLTα and RIPLTαLTβ−/− pancreata.
Figure 6
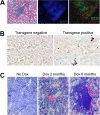
Lymphatic vessels are apparent before obvious TLOs. (A) H&E, DAPI, B220, and LYVE-1 staining in the kidney of a RIPLTα mouse (40× objective; Zeiss Axioscope). LYVE-1 positive lymphatic vessels (red) are within and around B cell regions (green) of the TLO. (B) In situ hybridization reveals LTα expression (dark purple) in the convoluted tubules of a RIPLTαTetOn mouse kidney after 1 month of doxycycline feeding (20× objective; Zeiss Axioscope). (C) Merge of bright field staining for hematoxylin and dark field for LYVE-1 positive vessels (red) reveals lymphatic vessels within kidney parenchyma after 2 months of doxycycline feeding before apparent extensive leukocytic infiltration. At 6 months, TLOs are apparent in the kidney with more obvious lymphatic vessels Cy-2 green LYVE-1 staining has been digitally colorized red to enhance contrast against hematoxylin (original magnification ×40; Zeiss Axioscope).
Figure 7
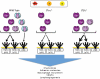
Working model of the roles of the LT/TNF family members in lymphangiogenesis in inflammation. The putative cellular origin of cytokines of the LT/TNF family is indicated at the top of the figure. In WT mice, all forms of the cytokines are indicated: TNFα3, LTα3, and LTα1β2. LTα2β1 is not included in the figure. The receptors for the various forms of the cytokines are indicated. LTα−/− mice produce only TNFα3. Even though these mice make LTβ mRNA, no protein is assembled on the cell surface due to the requirement of LTα for the cell surface expression of LTβ. TNFα3 and LTα3 are produced in LTβ−/− mice. The absence of LTβ allows more LTα to assemble as the LTα3 homotrimer, and is an explanation for the more intense inflammation in the absence of LTβ. TNFα3 and LTα3, working through the TNF receptors induce chemokines and inflammatory vascular adhesion molecules that then induce the accumulation of additional cells that can produce factors, such as VEGFs capable of inducing lymphangiogenesis. It is also possible that LTα3 and TNFα3 induce VEGFs directly from stromal cells and could also have direct effects on lymphatic endothelial cells.
Similar articles
- Distinct contributions of TNF and LT cytokines to the development of dendritic cells in vitro and their recruitment in vivo.
Abe K, Yarovinsky FO, Murakami T, Shakhov AN, Tumanov AV, Ito D, Drutskaya LN, Pfeffer K, Kuprash DV, Komschlies KL, Nedospasov SA. Abe K, et al. Blood. 2003 Feb 15;101(4):1477-83. doi: 10.1182/blood.V101.4.1477. Blood. 2003. PMID: 12560241 - Membrane lymphotoxin contributes to anti-leishmanial immunity by controlling structural integrity of lymphoid organs.
Wilhelm P, Riminton DS, Ritter U, Lemckert FA, Scheidig C, Hoek R, Sedgwick JD, Körner H. Wilhelm P, et al. Eur J Immunol. 2002 Jul;32(7):1993-2003. doi: 10.1002/1521-4141(200207)32:7<1993::AID-IMMU1993>3.0.CO;2-F. Eur J Immunol. 2002. PMID: 12115620 - Abnormal development of secondary lymphoid tissues in lymphotoxin beta-deficient mice.
Alimzhanov MB, Kuprash DV, Kosco-Vilbois MH, Luz A, Turetskaya RL, Tarakhovsky A, Rajewsky K, Nedospasov SA, Pfeffer K. Alimzhanov MB, et al. Proc Natl Acad Sci U S A. 1997 Aug 19;94(17):9302-7. doi: 10.1073/pnas.94.17.9302. Proc Natl Acad Sci U S A. 1997. PMID: 9256477 Free PMC article. - Biology and signal transduction pathways of the Lymphotoxin-αβ/LTβR system.
Remouchamps C, Boutaffala L, Ganeff C, Dejardin E. Remouchamps C, et al. Cytokine Growth Factor Rev. 2011 Oct-Dec;22(5-6):301-10. doi: 10.1016/j.cytogfr.2011.11.007. Cytokine Growth Factor Rev. 2011. PMID: 22152226 Review. - Dissecting the role of lymphotoxin in lymphoid organs by conditional targeting.
Tumanov AV, Grivennikov SI, Shakhov AN, Rybtsov SA, Koroleva EP, Takeda J, Nedospasov SA, Kuprash DV. Tumanov AV, et al. Immunol Rev. 2003 Oct;195:106-16. doi: 10.1034/j.1600-065x.2003.00071.x. Immunol Rev. 2003. PMID: 12969314 Review.
Cited by
- The role of non-hematopoietic stromal cells in the persistence of inflammation.
Barone F, Nayar S, Buckley CD. Barone F, et al. Front Immunol. 2013 Jan 14;3:416. doi: 10.3389/fimmu.2012.00416. eCollection 2012. Front Immunol. 2013. PMID: 23335923 Free PMC article. - Chronic Inflammation: A Common Promoter in Tertiary Lymphoid Organ Neogenesis.
Luo S, Zhu R, Yu T, Fan H, Hu Y, Mohanta SK, Hu D. Luo S, et al. Front Immunol. 2019 Dec 18;10:2938. doi: 10.3389/fimmu.2019.02938. eCollection 2019. Front Immunol. 2019. PMID: 31921189 Free PMC article. Review. - Lymphatic vessel function in head and neck inflammation.
Truman LA, A-Gonzalez N, Bentley KL, Ruddle NH. Truman LA, et al. Lymphat Res Biol. 2013 Sep;11(3):187-92. doi: 10.1089/lrb.2013.0013. Lymphat Res Biol. 2013. PMID: 24044758 Free PMC article. - PI3K p110δ is expressed by gp38(-)CD31(+) and gp38(+)CD31(+) spleen stromal cells and regulates their CCL19, CCL21, and LTβR mRNA levels.
Zotes TM, Spada R, Mulens V, Pérez-Yagüe S, Sorzano CO, Okkenhaug K, Carrera AC, Barber DF. Zotes TM, et al. PLoS One. 2013 Aug 29;8(8):e72960. doi: 10.1371/journal.pone.0072960. eCollection 2013. PLoS One. 2013. PMID: 24009720 Free PMC article. - Lymphotoxin α revisited: general features and implications in rheumatoid arthritis.
Calmon-Hamaty F, Combe B, Hahne M, Morel J. Calmon-Hamaty F, et al. Arthritis Res Ther. 2011 Jul 26;13(4):232. doi: 10.1186/ar3376. Arthritis Res Ther. 2011. PMID: 21861866 Free PMC article. Review.
References
- Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140(4):460–476. - PubMed
- Karkkainen MJ, Haiko P, Sainio K, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5(1):74–80. - PubMed
- Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98(6):769–778. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01CA82923/CA/NCI NIH HHS/United States
- P01 HL024136/HL/NHLBI NIH HHS/United States
- R01 CA082923/CA/NCI NIH HHS/United States
- R00 CA137167/CA/NCI NIH HHS/United States
- R01 CA016885/CA/NCI NIH HHS/United States
- R01 HL059157/HL/NHLBI NIH HHS/United States
- R01HL59157/HL/NHLBI NIH HHS/United States
- K99CA137167/CA/NCI NIH HHS/United States
- K99 CA137167-02/CA/NCI NIH HHS/United States
- R01 DK057731/DK/NIDDK NIH HHS/United States
- P01 HL24136/HL/NHLBI NIH HHS/United States
- R56 CA016885/CA/NCI NIH HHS/United States
- K99 CA137167/CA/NCI NIH HHS/United States
- P30 041942/PHS HHS/United States
- R01DK 57731/DK/NIDDK NIH HHS/United States
- R01CA085149/CA/NCI NIH HHS/United States
- CA 16885/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous