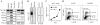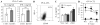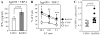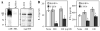DC-HIL/glycoprotein Nmb promotes growth of melanoma in mice by inhibiting the activation of tumor-reactive T cells - PubMed (original) (raw)
DC-HIL/glycoprotein Nmb promotes growth of melanoma in mice by inhibiting the activation of tumor-reactive T cells
Mizuki Tomihari et al. Cancer Res. 2010.
Abstract
DC-HIL/glycoprotein nmb (Gpnmb) expressed on antigen-presenting cells attenuates T-cell activation by binding to syndecan-4 (SD-4) on activated T cells. Because DC-HIL/Gpnmb is expressed abundantly by mouse and human melanoma lines, we posited that melanoma-associated DC-HIL/Gpnmb exerts similar inhibitory function on melanoma-reactive T cells. We generated small interfering RNA-transfected B16F10 melanoma cells to completely knock down DC-HIL/Gpnmb expression, with no alteration in cell morphology, melanin synthesis, or MHC class I expression. This knockdown had no effect on B16F10 proliferation in vitro or entry into the cell cycle following growth stimulation, but it markedly reduced the growth of these cells in vivo following their s.c. injection into syngeneic immunocompetent (but not immunodeficient) mice. This reduction in tumor growth was due most likely to an augmented capacity of DC-HIL-knocked down B16F10 cells (compared with controls) to activate melanoma-reactive T cells as documented in vitro and in mice. Whereas DC-HIL knockdown had no effect on susceptibility of melanoma to killing by cytotoxic T cells, blocking SD-4 function enhanced the reactivity of CD8(+) T cells to melanoma-associated antigens on parental B16F10 cells. Using an assay examining the spread to the lung following i.v. injection, DC-HIL-knocked down cells produced lung foci at similar numbers compared with that produced by control cells, but the size of the former foci was significantly smaller than the latter. We conclude that DC-HIL/Gpnmb confers upon melanoma the ability to downregulate the activation of melanoma-reactive T cells, thereby allowing melanoma to evade immunologic recognition and destruction. As such, the DC-HIL/SD-4 pathway is a potentially useful target for antimelanoma immunotherapy.
(c)2010 AACR.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figures
Figure 1. Expression of DC-HIL by B16F10 melanoma and characterization of DC-HIL-knocked-down cells
Expression of DC-HIL and other co-inhibitory ligands: (A) Total RNA was isolated from B16F10 melanoma cells untreated or treated with IFN-γ (1,000 U/ml) plus PMA (10 ng/ml) for 16 h or from BM-DC and examined by RT-PCR for mRNA expression of different co-inhibitory ligands and β-actin. PCR without cDNA (No cDNA) served as negative control. Characterization of DC-HIL-knocked-down B16F10 cells: B16F10 cells transfected with control lentivector (C-B16) or DC-HIL-targeted shRNA-lentivector (Kd-B16) were assessed for protein expression (B), in vitro proliferation (C), and subjected to cell cycle analysis (D). B, Whole cell extracts prepared from B16F10 transfectants were assayed by Western blotting for protein expression of DC-HIL or β-actin using UTX-103 anti-DC-HIL or anti-β-actin Ab. C, Synchronized B16F10 transfectants (1 × 104 cells/well) were allowed to grow in vitro, and proliferation measured by MTT assay (mean ± sd, n=3). D, After starving in serum-free media, B16F10 transfectants were stimulated for growth by FCS and labeled with BrdU, and then stained with APC-anti-BrdU Ab and 7-AAD (total DNA content), followed by flow cytometry. Data are shown as dot plots of BrdU vs. 7-AAD. All data are representative of at least 2 independent experiments.
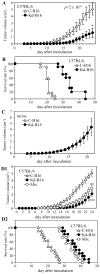
Figure 2. Knockdown of DC-HIL expression reduces growth of B16F10 melanoma in syngeneic wild-type (but not nu/nu) mice
Control (C-B16) or DC-HIL-knockdown B16F10 cells (Kd-B16) were inoculated s.c. into the right flank of C57BL/6 mice (12 mice/group, A and B) or nu/nu mice (5 mice/group, C), and tumor volume measured daily. Survival rate of C57BL/6 mice injected with B16F10 transfectants is shown (B). The two transfectants were also mixed (each 2 × 105 cells) and examined for tumor growth in C57BL/6 mice (n=10) (D1) and survival rate (D2). Statistical analysis (Student’s t test) of these two groups on days 13–21 indicates _p_=2 × 10−8 (A). ** (_p_= 1.5 × 10−8) and *** (_p_= 0.001) on day 24 document statistical significance of the mixed cells’ effect compared to C-B16 alone and Kd-B16 alone, respectively. Second experiment showed similar results.
Figure 3. Blockade of DC-HIL/SD-4 pathway leads to enhanced T cell immunogenicity
A, OVA-specific CD8+ T cells were stimulated by coculturing with OVA-pulsed/MMC-treated B16F10 transfectants and T cell activation assessed by production of IL-2 and IFN-γ. Statistical significance is denoted by * _p_=0.05 and ** _p_=0.01, as compared with production by T cells treated with C-B16 cells. B, Anti-OVA CTL were generated by immunization and subsequent in vitro stimulation and examined by flowcytometry for surface expression of SD-4 and CD69 (activation marker). C, OVA-specific CD8+ T cells were stimulated by OVA-pulsed/MMC-treated B16F10 melanoma in the absence (None) or presence of control IgG or anti-SD-4 mAb at different doses. T cell activation was assessed by IL-2 production. D, Anti-OVA CTL were allowed to kill target cells at varying E:T ratios. Target cells include OVA-pulsed Kd-B16 (• with solid lines), OVA-pulsed C-B16 (♠ with solid lines), untreated Kd-B16 (• with dashed lines), and untreated C-B16 cells (♠ with dashed lines). Statistical significance; *, _p_=0.20; **_p=_0.18, and *** _p_=0.58. Cytotoxicity is expressed as % of lysis. All data are representative of 3 separate experiments.
Figure 4. DC-HIL knockdown enhances capacity to stimulate anti-TAA T cells
A, Anti-hgp100/TRP-2 CTL were stimulated by C-B16 or Kd-B16 melanoma cells and IFN-γ production measured. B, These CTL were cocultured with 51Cr-labeled B16F10 transfectants or EL-4 as H-2-mismatached control, at varying E:T ratios. Statistical significance scores are denoted by * (_p_=0.59), ** (_p_=0.69), and *** (_p_=0.77). C, Two weeks after C57BL/6 mice (n=7) were tumor challenged with B16F10 transfectants, draining LN cells were prepared from treated mice and restimulated in vitro with γ-irradiated syngeneic spleen cells pulsed with hgp100 and TRP-2 peptides. Finally, IFN-γ-producing cells were counted by ELISPOT per 2 × 106 LN cells.
Figure 5. DC-HIL+ exsosomes from B16F10 cells inhibit T cell activation triggered by TAA on melanoma
A, Exosomes prepared from C-B16 or Kd-B16 cells were examined by immunoblotting for expression of DC-HIL and gp100 (as a melanosomal marker). B, These exosomes were added to the coculture of activated CD8+ T cells (from pmel-1 TCR-transgenic mice) and MMC-treated B16F10 cells. T cell activation was measured by production of IL-2 (left) or IFN-γ (right panel) (mean ±sd, n=3. * _p_>0.001).
Figure 6. Capacity of B16F10 transfectants to spread to lung and grow in the lung foci
C57BL/6 mice (n=6 for Exp 1 and n=5 for Exp 2) were given B16F10 transfectants by i.v. injection. 14 (Exp 1) or 11 (Exp 2) days post-injection, lungs were procured; weight, number of metastatic foci, total melanin content, and melanin content per focus were measured. Statistical significance (p value) is shown. Photographs of lungs from mice injected with B16F10 transfectants are shown on the right, with a scale bar of 50 mm. Images of some foci are enlarged.
Similar articles
- Syndecan-4 mediates the coinhibitory function of DC-HIL on T cell activation.
Chung JS, Dougherty I, Cruz PD Jr, Ariizumi K. Chung JS, et al. J Immunol. 2007 Nov 1;179(9):5778-84. doi: 10.4049/jimmunol.179.9.5778. J Immunol. 2007. PMID: 17947650 - The DC-HIL/syndecan-4 pathway inhibits human allogeneic T-cell responses.
Chung JS, Bonkobara M, Tomihari M, Cruz PD Jr, Ariizumi K. Chung JS, et al. Eur J Immunol. 2009 Apr;39(4):965-74. doi: 10.1002/eji.200838990. Eur J Immunol. 2009. PMID: 19350579 Free PMC article. - DC-HIL is a negative regulator of T lymphocyte activation.
Chung JS, Sato K, Dougherty II, Cruz PD Jr, Ariizumi K. Chung JS, et al. Blood. 2007 May 15;109(10):4320-7. doi: 10.1182/blood-2006-11-053769. Epub 2007 Feb 6. Blood. 2007. PMID: 17284525 Free PMC article. - DC-HIL/Gpnmb Is a Negative Regulator of Tumor Response to Immune Checkpoint Inhibitors.
Chung JS, Ramani V, Kobayashi M, Fattah F, Popat V, Zhang S, Cruz PD Jr, Gerber DE, Ariizumi K. Chung JS, et al. Clin Cancer Res. 2020 Mar 15;26(6):1449-1459. doi: 10.1158/1078-0432.CCR-19-2360. Epub 2019 Dec 10. Clin Cancer Res. 2020. PMID: 31822499 - The DC-HIL/syndecan-4 pathway regulates autoimmune responses through myeloid-derived suppressor cells.
Chung JS, Tamura K, Akiyoshi H, Cruz PD Jr, Ariizumi K. Chung JS, et al. J Immunol. 2014 Mar 15;192(6):2576-84. doi: 10.4049/jimmunol.1301857. Epub 2014 Feb 10. J Immunol. 2014. PMID: 24516197 Free PMC article.
Cited by
- Melanoma-derived conditioned media efficiently induce the differentiation of monocytes to macrophages that display a highly invasive gene signature.
Wang T, Ge Y, Xiao M, Lopez-Coral A, Azuma R, Somasundaram R, Zhang G, Wei Z, Xu X, Rauscher FJ 3rd, Herlyn M, Kaufman RE. Wang T, et al. Pigment Cell Melanoma Res. 2012 Jul;25(4):493-505. doi: 10.1111/j.1755-148X.2012.01005.x. Pigment Cell Melanoma Res. 2012. PMID: 22498258 Free PMC article. - Glycoprotein nonmetastatic melanoma protein B: A key mediator and an emerging therapeutic target in autoimmune diseases.
Tsou PS, Sawalha AH. Tsou PS, et al. FASEB J. 2020 Jul;34(7):8810-8823. doi: 10.1096/fj.202000651. Epub 2020 May 23. FASEB J. 2020. PMID: 32445534 Free PMC article. Review. - Beneficial impact of Gpnmb and its significance as a biomarker in nonalcoholic steatohepatitis.
Katayama A, Nakatsuka A, Eguchi J, Murakami K, Teshigawara S, Kanzaki M, Nunoue T, Hida K, Wada N, Yasunaka T, Ikeda F, Takaki A, Yamamoto K, Kiyonari H, Makino H, Wada J. Katayama A, et al. Sci Rep. 2015 Nov 19;5:16920. doi: 10.1038/srep16920. Sci Rep. 2015. PMID: 26581806 Free PMC article. - A locked, dimeric CXCL12 variant effectively inhibits pulmonary metastasis of CXCR4-expressing melanoma cells due to enhanced serum stability.
Takekoshi T, Ziarek JJ, Volkman BF, Hwang ST. Takekoshi T, et al. Mol Cancer Ther. 2012 Nov;11(11):2516-25. doi: 10.1158/1535-7163.MCT-12-0494. Epub 2012 Aug 6. Mol Cancer Ther. 2012. PMID: 22869557 Free PMC article. - Expression pattern and prognostic impact of glycoprotein non-metastatic B (GPNMB) in triple-negative breast cancer.
Huang YH, Chu PY, Chen JL, Huang CT, Huang CC, Tsai YF, Wang YL, Lien PJ, Tseng LM, Liu CY. Huang YH, et al. Sci Rep. 2021 Jun 9;11(1):12171. doi: 10.1038/s41598-021-91588-3. Sci Rep. 2021. PMID: 34108545 Free PMC article.
References
- Rivoltini L, Carrabba M, Huber V, Castelli C, Novellino L, Dalerba P, et al. Immunity to cancer: attack and escape in T lymphocyte-tumor cell interaction. Immunol Rev. 2002 Oct;188:97–113. - PubMed
- Chouaib S, Asselin-Paturel C, Caignard A, Blay JY. The host-tumor immune conflict: from immunosuppression to resistance and destruction. Immunol Today. 1997 Oct;18(10):493–7. - PubMed
- Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29–53. - PubMed
- Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999 Dec;5(12):1365–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI064927/AI/NIAID NIH HHS/United States
- R01 AI064927-06/AI/NIAID NIH HHS/United States
- R56 AI064927/AI/NIAID NIH HHS/United States
- AI064927/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials