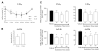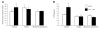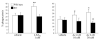FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin-derived peptide-stimulated phagocytosis - PubMed (original) (raw)
FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin-derived peptide-stimulated phagocytosis
Paola Maderna et al. FASEB J. 2010 Nov.
Abstract
Lipoxins (LXs) are endogenously produced eicosanoids with well-described anti-inflammatory and proresolution activities, stimulating nonphlogistic phagocytosis of apoptotic cells by macrophages. LXA(4) and the glucocorticoid-derived annexin A1 peptide (Ac2-26) bind to a common G-protein-coupled receptor, termed FPR2/ALX. However, direct evidence of the involvement of FPR2/ALX in the anti-inflammatory and proresolution activity of LXA(4) is still to be investigated. Here we describe FPR2/ALX trafficking in response to LXA(4) and Ac2-26 stimulation. We have transfected cells with HA-tagged FPR2/ALX and studied receptor trafficking in unstimulated, LXA(4) (1-10 nM)- and Ac2-26 (30 μM)-treated cells using multiple approaches that include immunofluorescent confocal microscopy, immunogold labeling of cryosections, and ELISA and investigated receptor trafficking in agonist-stimulated phagocytosis. We conclude that PKC-dependent internalization of FPR2/ALX is required for phagocytosis. Using bone marrow-derived macrophages (BMDMs) from mice in which the FPR2/ALX ortholog Fpr2 had been deleted, we observed the nonredundant function for this receptor in LXA(4) and Ac2-26 stimulated phagocytosis of apoptotic neutrophils. LXA(4) stimulated phagocytosis 1.7-fold above basal (P<0.001) by BMDMs from wild-type mice, whereas no effect was found on BMDMs from Fpr2(-/-) mice. Similarly, Ac2-26 stimulates phagocytosis by BMDMs from wild-type mice 1.5-fold above basal (P<0.05). However, Ac2-26 failed to stimulate phagocytosis by BMDMs isolated from Fpr2(-/-) mice relative to vehicle. These data reveal novel and complex mechanisms of the FPR2/ALX receptor trafficking and functionality in the resolution of inflammation.
Figures
Figure 1
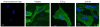
LXA4- and Ac2–26-induced internalization of FPR2/ALX: confocal microscopy. Hela cells transiently expressing HA-tagged FPR2/ALX plated on poly-
l
-lysine-coated coverslips were treated with vehicle (EtOH), LXA4 (1 nM), or Ac2–26 (30 μM) for 15 min. Cells were fixed with paraformaldehyde, permeabilized with Triton X-100, and subjected to detection of receptor using an anti-HA primary antibody, followed by a Oregon Green-conjugated secondary antibody. Confocal microscopy images representative of ≥5 experiments are shown. Image of untransfected cells is shown as negative control.
Figure 2
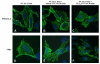
Constitutive and agonist-dependent internalization of FPR2/ALX and FPR3. Hela cells transiently transfected with HA-tagged FPR2/ALX (A–C) or FPR3 (D–F) were labeled at 4°C for 1 h with anti HA primary antibody prior to incubate the cells at 37°C in the presence of vehicle (EtOh) or LXA4 (1 nM) for 30 min. Cells were then fixed with paraformaldehyde and receptor was stained with Oregon Green anti-mouse secondary antibody. Confocal microscopy images were acquired with an ×65 oil lens. Data are representative of ≥3 different experiments.
Figure 3
Cryosectioning and immunogold labeling of FPR2/ALX. Hela cells transiently transfected with HA-tagged FPRL1/ALX were treated with vehicle (EtOH; A) or LXA4 (1 nM) for 5 min (B), 15 min (C), and 30 min (D). Cells were fixed with paraformaldehyde/glutaraldehyde and cryosectioned (60-nm sections). Sections were incubated with anti-HA antibody, followed by an anti-mouse 10-nm gold-conjugated antibody, and contrasted by incubation in uranyl acetate in methyl cellulose. View: ×65,000 (A, D); ×100,000 (B, C).
Figure 4
Quantification of LXA4- and Ac2–26-induced internalization of FPR2/ALX by ELISA and FACS. A, B) Hela cells transiently expressing HA-tagged FPR2/ALX were stimulated for the indicated times with 1 nM LXA4 (A) or 30 μM Ac2–26 (B). The loss of cell surface HA expression, index of receptor internalization, was measured by ELISA, as described in Materials and Methods. Data represent means ±se; n = 4–6 *P < 0.05 vs. vehicle. C) Isolated human PMNs or HEK cells transfected with the FPR2 receptor were incubated with vehicle, 10 μM Ac2–26, or 1 nM LXA4 for the indicated times. At the end of each point, further receptor movement was inhibited. Following incubation with a mouse monoclonal anti-human FPR2 antibody or an isotype, control cells were washed and incubated for a further 30 min at 4°C with an anti-mouse FITC-conjugated secondary antibody. At least 10,000 events were analyzed using a FACSCalibur flow cytometer and CellQuest software. Surface protein expression was recorded as MFI units measured in the FL1 green channel. Data are means ±
se
from ≥4 independent experiments for both PMNs and HEK cells.
Figure 5
Protein kinase C is involved in the LXA4-induced internalization of FPR2/ALX. Hela cells transiently expressing HA-tagged FPR2/ALX were pretreated for 30 min with vehicle (Veh), BisI (10 μM), wortmannin (100 nM), or genistein (100 μM), and then stimulated with LXA4 (1 nM) for 15 min, and loss of cell surface receptors was assessed by ELISA (_n_=3). *P < 0.05 vs. vehicle.
Figure 6
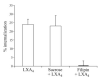
Disruption of lipid raft affected FPR2/ALX internalization. Hela cells transiently expressing HA-tagged FPR2/ALX were pretreated with sucrose (0.45 M) or filipin (5 μg/ml) for 30 min prior to treatment with LXA4 (1 nM) for 15 min. Loss of cell surface receptors was assessed by ELISA (_n_=3).
Figure 7
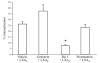
Disruption of lipid raft affected LXA4-induced phagocytosis. Differentiated THP-1 cells were pretreated with filipin (5 μg/ml) for or methyl-β-cyclodextrin (10 mM) prior treatment with vehicle (EtOH) or LXA4 (1 nM) for 15 min. A) Opsonized fluorescent zymosan was added (ratio 1:5), and phagocytosis was allowed to proceed for 2 h. Internalized fluorescence associated with internalized zymosan was measured with a fluorescence plate reader. B) Apoptotic PMNs were added, and phagocytosis was allowed to proceed for 2 h. Phagocytosis was quantified as described in Materials and Methods. Data are expressed as mean ±
se
percentage of phagocytosis (_n_=4). * P < 0.05 vs. vehicle.
Figure 8
BMDMs from Fpr2−/− mice show defective phagocytosis of apoptotic PMNs. BMDMs isolated from WT or _Fpr2_−/− mice were exposed to vehicle, LXA4 (1 nM) or Ac2–26 (10–30 μM) before incubation with human apoptotic PMNs. Phagocytosis was allowed to proceed for 30 min. Phagocytosis was quantified my microscopy after staining for myeloperoxidase as described in Materials and Methods. Data are expressed as mean ±
se
percentage of phagocytosis (_n_=4). *P < 0.05, **P < 0.001, #P < 0.05 vs. vehicle.
Similar articles
- MicroRNA-181b regulates ALX/FPR2 receptor expression and proresolution signaling in human macrophages.
Pierdomenico AM, Recchiuti A, Simiele F, Codagnone M, Mari VC, Davì G, Romano M. Pierdomenico AM, et al. J Biol Chem. 2015 Feb 6;290(6):3592-600. doi: 10.1074/jbc.M114.592352. Epub 2014 Dec 11. J Biol Chem. 2015. PMID: 25505240 Free PMC article. - Evidence for an anti-inflammatory loop centered on polymorphonuclear leukocyte formyl peptide receptor 2/lipoxin A4 receptor and operative in the inflamed microvasculature.
Brancaleone V, Dalli J, Bena S, Flower RJ, Cirino G, Perretti M. Brancaleone V, et al. J Immunol. 2011 Apr 15;186(8):4905-14. doi: 10.4049/jimmunol.1003145. Epub 2011 Mar 11. J Immunol. 2011. PMID: 21398608 Free PMC article. - Heterologously expressed formyl peptide receptor 2 (FPR2/ALX) does not respond to lipoxin A₄.
Hanson J, Ferreirós N, Pirotte B, Geisslinger G, Offermanns S. Hanson J, et al. Biochem Pharmacol. 2013 Jun 15;85(12):1795-802. doi: 10.1016/j.bcp.2013.04.019. Epub 2013 May 1. Biochem Pharmacol. 2013. PMID: 23643932 - The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo.
Chiang N, Serhan CN, Dahlén SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C. Chiang N, et al. Pharmacol Rev. 2006 Sep;58(3):463-87. doi: 10.1124/pr.58.3.4. Pharmacol Rev. 2006. PMID: 16968948 Review. - Treating neutrophilic inflammation in COPD by targeting ALX/FPR2 resolution pathways.
Bozinovski S, Anthony D, Anderson GP, Irving LB, Levy BD, Vlahos R. Bozinovski S, et al. Pharmacol Ther. 2013 Dec;140(3):280-9. doi: 10.1016/j.pharmthera.2013.07.007. Epub 2013 Jul 21. Pharmacol Ther. 2013. PMID: 23880288 Review.
Cited by
- Synthesis and Biological Evaluation of Bicyclo[1.1.1]pentane-Containing Aromatic Lipoxin A4 Analogues.
Owen B, de Gaetano M, Gaffney A, Godson C, Guiry PJ. Owen B, et al. Org Lett. 2022 Aug 19;24(32):6049-6053. doi: 10.1021/acs.orglett.2c02345. Epub 2022 Aug 8. Org Lett. 2022. PMID: 35938947 Free PMC article. - Novel Role for the AnxA1-Fpr2/ALX Signaling Axis as a Key Regulator of Platelet Function to Promote Resolution of Inflammation.
Senchenkova EY, Ansari J, Becker F, Vital SA, Al-Yafeai Z, Sparkenbaugh EM, Pawlinski R, Stokes KY, Carroll JL, Dragoi AM, Qin CX, Ritchie RH, Sun H, Cuellar-Saenz HH, Rubinstein MR, Han YW, Orr AW, Perretti M, Granger DN, Gavins FNE. Senchenkova EY, et al. Circulation. 2019 Jul 23;140(4):319-335. doi: 10.1161/CIRCULATIONAHA.118.039345. Epub 2019 Jun 3. Circulation. 2019. PMID: 31154815 Free PMC article. - Nonredundant protective properties of FPR2/ALX in polymicrobial murine sepsis.
Gobbetti T, Coldewey SM, Chen J, McArthur S, le Faouder P, Cenac N, Flower RJ, Thiemermann C, Perretti M. Gobbetti T, et al. Proc Natl Acad Sci U S A. 2014 Dec 30;111(52):18685-90. doi: 10.1073/pnas.1410938111. Epub 2014 Dec 15. Proc Natl Acad Sci U S A. 2014. PMID: 25512512 Free PMC article. - Lipoxin A4 activates ALX/FPR2 receptor to regulate conjunctival goblet cell secretion.
Hodges RR, Li D, Shatos MA, Bair JA, Lippestad M, Serhan CN, Dartt DA. Hodges RR, et al. Mucosal Immunol. 2017 Jan;10(1):46-57. doi: 10.1038/mi.2016.33. Epub 2016 Apr 13. Mucosal Immunol. 2017. PMID: 27072607 Free PMC article. - Annexin A1 N-terminal derived Peptide ac2-26 exerts chemokinetic effects on human neutrophils.
Dalli J, Montero-Melendez T, McArthur S, Perretti M. Dalli J, et al. Front Pharmacol. 2012 Feb 28;3:28. doi: 10.3389/fphar.2012.00028. eCollection 2012. Front Pharmacol. 2012. PMID: 22403546 Free PMC article.
References
- Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostag. Leukot. Essent. Fatty Acids. 2005;73:141–162. - PubMed
- Maderna P, Godson C. Phagocytosis of apoptotic cells and the resolution of inflammation. Biochim. Biophys. Acta. 2003;1639:141–151. - PubMed
- Kieran NE, Maderna P, Godson C. Lipoxins: potential anti-inflammatory, proresolution, and antifibrotic mediators in renal disease. Kidney Int. 2004;65:1145–1154. - PubMed
- McMahon B, Godson C. Lipoxins: endogenous regulators of inflammation. Am. J. Physiol. Renal Physiol. 2004;286:F189–F201. - PubMed
- Maderna P, Godson C. Taking insult from injury: lipoxins and lipoxin receptor agonists and phagocytosis of apoptotic cells. Prostag. Leukot. Essent. Fatty Acids. 2005;73:179–187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources