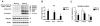Progressive accumulation of amyloid-beta oligomers in Alzheimer's disease and in amyloid precursor protein transgenic mice is accompanied by selective alterations in synaptic scaffold proteins - PubMed (original) (raw)
. 2010 Jul;277(14):3051-67.
doi: 10.1111/j.1742-4658.2010.07719.x. Epub 2010 Jun 22.
Leslie Crews, Kiren Ubhi, Lawrence Hansen, Anthony Adame, Anna Cartier, David Salmon, Douglas Galasko, Sarah Michael, Jeffrey N Savas, John R Yates, Charles Glabe, Eliezer Masliah
Affiliations
- PMID: 20573181
- PMCID: PMC2933033
- DOI: 10.1111/j.1742-4658.2010.07719.x
Progressive accumulation of amyloid-beta oligomers in Alzheimer's disease and in amyloid precursor protein transgenic mice is accompanied by selective alterations in synaptic scaffold proteins
Emiley Pham et al. FEBS J. 2010 Jul.
Abstract
The cognitive impairment in patients with Alzheimer's disease is closely associated with synaptic loss in the neocortex and limbic system. Although the neurotoxic effects of aggregated amyloid-beta oligomers in Alzheimer's disease have been studied extensively in experimental models, less is known about the characteristics of these aggregates across the spectrum of Alzheimer's disease. In this study, postmortem frontal cortex samples from controls and patients with Alzheimer's disease were fractionated and analyzed for levels of oligomers and synaptic proteins. We found that the levels of oligomers correlated with the severity of cognitive impairment (blessed information-memory-concentration score and mini-mental state examination) and with the loss of synaptic markers. Reduced levels of the synaptic vesicle protein, vesicle-associated membrane protein-2, and the postsynaptic protein, postsynaptic density-95, correlated with the levels of oligomers in the various fractions analyzed. The strongest associations were found with amyloid-beta dimers and pentamers. Co-immunoprecipitation and double-labeling experiments supported the possibility that amyloid-beta and postsynaptic density-95 interact at synaptic sites. Similarly, in transgenic mice expressing high levels of neuronal amyloid precursor protein, amyloid-beta co-immunoprecipitated with postsynaptic density-95. This was accompanied by a decrease in the levels of the postsynaptic proteins Shank1 and Shank3 in patients with Alzheimer's disease and in the brains of amyloid precursor protein transgenic mice. In conclusion, this study suggests that the presence of a subpopulation of amyloid-beta oligomers in the brains of patients with Alzheimer's disease might be related to alterations in selected synaptic proteins and cognitive impairment.
Figures
Fig. 1
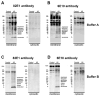
Comparative immunoblot analysis for APP/Aβ in the frontal cortex of control and AD patients. Samples were fractionated into membrane and cytosolic fractions and probed with anti-Aβ antibodies (82E1 and 6E10). A & B. In samples homogenized using Buffer A, compared to non-demented controls, in AD samples multiple bands representing Aβ monomers and multimers were identified at molecular weights ranging from 4 to 28 kDa bands in the membrane fraction. C & D. In samples homogenized using Buffer B, compared to non-demented control, in AD samples the majority of the Aβ was identified as a 4 kDa band in the membrane fraction.
Fig. 2
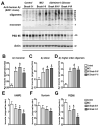
Analysis of the Aβ and synaptic protein bands detected by immunoblot in control and AD brain samples. Samples were homogenized using Buffer A and probed with antibodies against Aβ (82E1) and synaptic proteins (VAMP2, syntaxin, PSD95). A. Representative western blot with the membrane fractions from controls, MCI and AD cases displaying bands corresponding to Aβ monomer (4 kDa) and multimers (8-28 kDa) and PSD95 (95 kDa). B-D. Semi-quantitative analysis of the bands representing Aβ monomer (4 kDa) (B), dimer (8 kDa) (C) and higher order oligomers (12-28 kDa) (D) showing a progressive increase in AD cases. E-G. Semi-quantitative analysis of immunoblots for VAMP2 (E), syntaxin (F), and PSD95 (G) showing a reduction in immunoreactivity in AD cases. N=5 cases per group, *P<0.05 compared to non-demented control by one-way ANOVA with post-hoc Dunnett’s test.
Fig. 3
Immunohistochemical analysis of the patterns of Aβ immunoreactivity (82E1) in AD cases and APP tg samples. For panels A-F, vibratome sections were immunolabeled with an anti-Aβ antibody (82E1) and reacted with DAB. For panels G-L, vibratome sections were double-immunolabeled with an anti- Aβ antibody (82E1, green channel) and PSD95 (red channel). A-C. Compared to non-demented controls (A), in the frontal cortex of MCI cases Aβ was detected as discrete granular structures (arrows, B). In advanced AD cases, the antibody detected abundant plaques (C). D-F. Compared to nontg controls (D), in the frontal cortex of APP tg mice the anti-Aβ antibody detected discrete diffuse structures (arrows, E) in the neuropil as well as fibrillar mature plaques (F). G-I. In mild AD cases the discrete Aβ -positive granular structures co-localized with PSD95 (arrows). J-L. In APP tg mice the diffuse Aβ -positive structures co-localized with PSD95 (arrows). Scale bar in panel A equals 50 μm in panels A-F; scale bar in panel G equals 20 μm in panels G-L.
Fig. 4
Analysis of the Aβ and synaptic protein bands detected by immunoblot in APP tg mice. Samples were homogenized using Buffer A and probed with antibodies against Aβ (82E1) and synaptic proteins (VAMP2, syntaxin, PSD95). All panels are from the brains of 6-month old mice. A. Representative western blot of the membrane fractions from 6-month old nontg control and APP tg mice displaying bands corresponding to Aβ monomer (4 kDa) and multimers (8-28 kDa) and PSD95 (95 kDa). B. Semi-quantitative analysis of the bands representing Aβ monomer (4 kDa), dimer (8 kDa) and higher order oligomers (12-28 kDa). C. Semi-quantitative analysis of the immunoblots for VAMP2, syntaxin, PSD95. N=8 mice per group, *P<0.01 compared to nontg control by unpaired, two-tailed Student’s t-test.
Fig. 5
Co-immunoprecipitation studies for Aβ and PSD95 in AD cases and APP tg mice. Samples from the frontal cortex of human non-demented control and AD cases, or from the brains of nontg and APP tg mice were homogenized with Buffer A and membrane fractions were processed for immunoprecipitation. A. Samples from the brains of control and AD cases were immunoprecipitated (IP) with an anti- Aβ antibody (82E1), then analyzed by western blot (WB) with an antibody against PSD95. The reactive band was more intense in the AD cases (arrow); no reactive bands at 95 kDa were observed under control conditions. B. Semi-quantitative analysis of the co-immunoprecipitated band showed higher levels in AD cases. C. Mouse brain cortex samples from 6-month old animals were immunoprecipitated with anti- Aβ antibody (82E1), then analyzed by western blot with an antibody against PSD95. The reactive band was more intense in the APP tg mice (arrow). D. Semi-quantitative analysis of the co-immunoprecipitated band showed higher levels in APP tg mice. E. Mouse brain cortex samples from 6-month old animals were immunoprecipitated with anti-PSD95 antibody, then analyzed by western blot with an antibody against Aβ (82E1). The reactive band was more intense in the APP tg mice (arrow). N=3 cases or mice per group, *P<0.05 compared to control by one-way ANOVA with post-hoc Dunnett’s test. F. Mouse brain cortex samples from 6-month old APP tg mice were immunoprecipitated with an antibody against Aβ (82E1) and the resulting co-precipitates were analyzed by mass spectroscopy. Both Abeta trypitic peptides were identified along with three PSD-95 peptides. Charge and XCorr score of the identified peptides are indicated.
Fig. 6
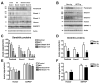
Analysis of the dendritic scaffold proteins by immunoblot in AD brains and APP tg mice. Panel A is from the frontal cortex of control, MCI and AD cases prepared with membrane fractions in Buffer A. Panel B is from nontg and APP tg cortex prepared with membrane fractions in Buffer A. A. Representative western blot analysis of human brain samples probed with antibodies against pan-Shank, Shank1, Shank3, Homer and SAPAP1. B. Representative western blot analysis of mouse brain samples probed with antibodies against pan-Shank, Shank1, Shank3, Homer and SAPAP1. C & D. Semi-quantitative analysis showing a reduction of Shank proteins in MCI and AD compared to non-demented control (C), and a similar reduction in APP tg mice compared to nontg controls (D). E & F. Semi-quantitative analysis showing no changes in Homer or SAPAP1 levels in diseased human (E) or tg mouse brains (F). N=5 cases per group for control, MCI and AD samples, *P<0.05 compared to non-demented controls by one-way ANOVA with post-hoc Dunnett’s test. N=8 mice per group for nontg and APP tg mice, *P<0.01 compared to nontg controls by unpaired, two-tailed Student’s t-test.
Fig. 7
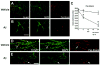
Double immunolabeling analysis for MAP2 and pan-Shank in primary neuronal cultures treated with conditioned media containing Aβ oligomers. Hippocampal neuronal cells from P1 mice were treated for 6 or 24 hrs with conditioned media from APP-expressing CHO cells (80 pM, a sublethal dose). Fixed cells on coverslips were immunolabeled with antibodies against MAP2 (green channel) and pan-Shank (red channel) and analyzed with a laser scanning confocal microscope. All images are from cells treated for 24 hrs; the graph represents data from both 6 and 24 hr timepoints. A & B. Confocal images showing neurons after 24 hrs of treatment with vehicle (A) or Aβ (B). Compared to vehicle-treated cells, Abtreatment resulted in a reduction in pan-Shank-positive punctae along the dendrites. C. Analysis of levels of pan-Shank and MAP2-immunoreactive structures after 6 and 24 hrs of treatment with vehicle or Aβ. D & E. Confocal images at higher power showing the detail of MAP2-labeled dendritic branches and pan-Shank-immunoreactive punctae (arrows) along the dendrites. Scale bar in panel B equals 20 μm for panels A & B; scale bar in panel E equals 10 μm for panels D & E. N=3 samples per condition, *P<0.05 compared to vehicle-treated controls by unpaired, two-tailed Student’s t-test.
Similar articles
- Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses.
Almeida CG, Tampellini D, Takahashi RH, Greengard P, Lin MT, Snyder EM, Gouras GK. Almeida CG, et al. Neurobiol Dis. 2005 Nov;20(2):187-98. doi: 10.1016/j.nbd.2005.02.008. Neurobiol Dis. 2005. PMID: 16242627 - Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers.
Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Laurén J, et al. Nature. 2009 Feb 26;457(7233):1128-32. doi: 10.1038/nature07761. Nature. 2009. PMID: 19242475 Free PMC article. - Human Brain-Derived Aβ Oligomers Bind to Synapses and Disrupt Synaptic Activity in a Manner That Requires APP.
Wang Z, Jackson RJ, Hong W, Taylor WM, Corbett GT, Moreno A, Liu W, Li S, Frosch MP, Slutsky I, Young-Pearse TL, Spires-Jones TL, Walsh DM. Wang Z, et al. J Neurosci. 2017 Dec 6;37(49):11947-11966. doi: 10.1523/JNEUROSCI.2009-17.2017. Epub 2017 Nov 3. J Neurosci. 2017. PMID: 29101243 Free PMC article. - Drebrin in Alzheimer's Disease.
Ishizuka Y, Hanamura K. Ishizuka Y, et al. Adv Exp Med Biol. 2017;1006:203-223. doi: 10.1007/978-4-431-56550-5_12. Adv Exp Med Biol. 2017. PMID: 28865022 Review. - Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior.
Selkoe DJ. Selkoe DJ. Behav Brain Res. 2008 Sep 1;192(1):106-13. doi: 10.1016/j.bbr.2008.02.016. Epub 2008 Feb 17. Behav Brain Res. 2008. PMID: 18359102 Free PMC article. Review.
Cited by
- Treadmill Exercise Facilitates Synaptic Plasticity in APP/PS1 Mice by Regulating Hippocampal AMPAR Activity.
Yu L, Li Y, Lv Y, Gu B, Cai J, Liu QS, Zhao L. Yu L, et al. Cells. 2024 Sep 25;13(19):1608. doi: 10.3390/cells13191608. Cells. 2024. PMID: 39404372 Free PMC article. - Local molecular and connectomic contributions of tau-related neurodegeneration.
Nabizadeh F; Alzheimer’s disease Neuroimaging Initiative (ADNI). Nabizadeh F, et al. Geroscience. 2024 Sep 30. doi: 10.1007/s11357-024-01339-1. Online ahead of print. Geroscience. 2024. PMID: 39343862 - Dexmedetomidine improves the circulatory dysfunction of the glymphatic system induced by sevoflurane through the PI3K/AKT/ΔFosB/AQP4 pathway in young mice.
Wang S, Yu X, Cheng L, Ren W, Wen G, Wu X, Lou H, Ren X, Lu L, Hermenean A, Yao J, Li B, Lu Y, Wu X. Wang S, et al. Cell Death Dis. 2024 Jun 25;15(6):448. doi: 10.1038/s41419-024-06845-w. Cell Death Dis. 2024. PMID: 38918408 Free PMC article. - MicroRNAs dysregulated in multiple sclerosis affect the differentiation of CG-4 cells, an oligodendrocyte progenitor cell line.
Perdaens O, Bottemanne P, van Pesch V. Perdaens O, et al. Front Cell Neurosci. 2024 Feb 29;18:1336439. doi: 10.3389/fncel.2024.1336439. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38486710 Free PMC article. - Misfolded protein oligomers: mechanisms of formation, cytotoxic effects, and pharmacological approaches against protein misfolding diseases.
Rinauro DJ, Chiti F, Vendruscolo M, Limbocker R. Rinauro DJ, et al. Mol Neurodegener. 2024 Feb 20;19(1):20. doi: 10.1186/s13024-023-00651-2. Mol Neurodegener. 2024. PMID: 38378578 Free PMC article. Review.
References
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. - PubMed
- DeKosky S, Scheff S. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. AnnNeurol. 1990;27:457–464. - PubMed
- DeKosky ST, Scheff SW, Styren SD. Structural correlates of cognition in dementia: quantification and assessment of synapse change. Neurodegeneration. 1996;5:417–421. - PubMed
- Sisodia SS, Price DL. Role of the beta-amyloid protein in Alzheimer’s disease. Faseb J. 1995;9:366–370. - PubMed
- Selkoe D. Amyloid β protein precursor and the pathogenesis of Alzheimer’s disease. Cell. 1989;58:611–612. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG11385/AG/NIA NIH HHS/United States
- R01 AG011385/AG/NIA NIH HHS/United States
- P50 AG005131/AG/NIA NIH HHS/United States
- P01 AG022074-080009/AG/NIA NIH HHS/United States
- R01 AG018440-05/AG/NIA NIH HHS/United States
- R01 AG018440/AG/NIA NIH HHS/United States
- R37 AG018440/AG/NIA NIH HHS/United States
- AG022074/AG/NIA NIH HHS/United States
- P50 AG005131-190023/AG/NIA NIH HHS/United States
- R37 AG011385/AG/NIA NIH HHS/United States
- AG5131/AG/NIA NIH HHS/United States
- AG18440/AG/NIA NIH HHS/United States
- P01 AG022074/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases