Non-germline genetically engineered mouse models for translational cancer research - PubMed (original) (raw)
Review
Non-germline genetically engineered mouse models for translational cancer research
Joerg Heyer et al. Nat Rev Cancer. 2010 Jul.
Abstract
Genetically engineered mouse models (GEMMs) of cancer have affected virtually all areas of cancer research. However, the accelerated discovery of new cancer genes emerging from large-scale cancer genomics and new chemical entities pouring from the drug discovery pipeline have strained the capacity of traditional germline mouse models to provide crucial insights. This Review introduces new approaches to modelling cancer, with emphasis on a growing collection of non-germline GEMMs (nGEMMs). These offer flexibility, speed and uniformity at reduced costs, thus paving the way for much needed throughput and practical preclinical therapeutic testing models.
Conflict of interest statement
Competing interests statement
The authors declare competing financial interests; see Web version for details.
Figures
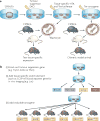
Figure 1. Conditional genetically engineered mouse models (GEMMs)
a | Activating Cre in a tissue-specific manner, by either viral delivery or Cre-oestrogen response element (ERE)-mediated tamoxifen administration. Examples are shown for lung cancer and ovarian cancer, and melanoma. b | Tetracycline (Tet)-mediated transgene inactivation or activation by doxycycline administration or withdrawal using the Tet-On or Tet-Off systems. Examples are shown for liver cancer and melanoma. fl, flox; lsl, lox-STOP-lox; rtTA, reverse transactivator; tTA, transactivator.
Figure 2. Chimeric model generation
a | Genetically modified embryonic stem cells are transduced ex vivo with conditional oncogenic transgenes, injected into blastocysts and implanted into pseudopregnant mice to generate chimeras. b | By activating the respective transgenes in a tissue-specific manner, multiple tumour types can be induced in these chimeras in the context of normal stromal tissue derived from the wild-type blastocysts. Image is modified, with permission, from Nature Biotechnology REF. © (2010) Macmillan Publishers Ltd. All rights reserved. DKO, double knockout; EGFR, epidermal growth factor receptor; Luc, luciferase; rtTA, reverse transactivator; Tet, tetracycline.
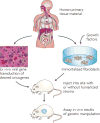
Figure 3. Human in mouse transplantation models
Pluripotent human stem cells are grown ex vivo on feeder cells and transduced with the oncogenic elements of interest. Without long culture times, the cells are injected into orthotopic sites on the mice with or without humanized stromal cells. Here, the tumour types of interest will develop from human cells within the context of the relevant mouse or human normal stroma.
Similar articles
- Advances in Making Cancer Mouse Models More Accessible and Informative through Non-Germline Genetic Engineering.
Murphy KC, Ruscetti M. Murphy KC, et al. Cold Spring Harb Perspect Med. 2024 Apr 1;14(4):a041348. doi: 10.1101/cshperspect.a041348. Cold Spring Harb Perspect Med. 2024. PMID: 37277206 Review. - Overview of genetically engineered mouse models of colorectal carcinoma to enable translational biology and drug development.
Roper J, Martin ES, Hung KE. Roper J, et al. Curr Protoc Pharmacol. 2014 Jun 16;65:14.29.1-10. doi: 10.1002/0471141755.ph1429s65. Curr Protoc Pharmacol. 2014. PMID: 24934606 - Genetically engineered mouse models in oncology research and cancer medicine.
Kersten K, de Visser KE, van Miltenburg MH, Jonkers J. Kersten K, et al. EMBO Mol Med. 2017 Feb;9(2):137-153. doi: 10.15252/emmm.201606857. EMBO Mol Med. 2017. PMID: 28028012 Free PMC article. Review. - Priceless GEMMs: genetically engineered mouse models for colorectal cancer drug development.
Roper J, Hung KE. Roper J, et al. Trends Pharmacol Sci. 2012 Aug;33(8):449-55. doi: 10.1016/j.tips.2012.05.001. Epub 2012 Jun 26. Trends Pharmacol Sci. 2012. PMID: 22739258 Review. - Translational therapeutics in genetically engineered mouse models of cancer.
Olive KP, Politi K. Olive KP, et al. Cold Spring Harb Protoc. 2014 Feb 1;2014(2):131-43. doi: 10.1101/pdb.top069997. Cold Spring Harb Protoc. 2014. PMID: 24492770
Cited by
- Effect of mutation order on myeloproliferative neoplasms.
Ortmann CA, Kent DG, Nangalia J, Silber Y, Wedge DC, Grinfeld J, Baxter EJ, Massie CE, Papaemmanuil E, Menon S, Godfrey AL, Dimitropoulou D, Guglielmelli P, Bellosillo B, Besses C, Döhner K, Harrison CN, Vassiliou GS, Vannucchi A, Campbell PJ, Green AR. Ortmann CA, et al. N Engl J Med. 2015 Feb 12;372(7):601-612. doi: 10.1056/NEJMoa1412098. N Engl J Med. 2015. PMID: 25671252 Free PMC article. - Making sense of cancer genomic data.
Chin L, Hahn WC, Getz G, Meyerson M. Chin L, et al. Genes Dev. 2011 Mar 15;25(6):534-55. doi: 10.1101/gad.2017311. Genes Dev. 2011. PMID: 21406553 Free PMC article. Review. - Chronic myeloid leukemia stem cells: targeting therapeutic implications.
Mojtahedi H, Yazdanpanah N, Rezaei N. Mojtahedi H, et al. Stem Cell Res Ther. 2021 Dec 18;12(1):603. doi: 10.1186/s13287-021-02659-1. Stem Cell Res Ther. 2021. PMID: 34922630 Free PMC article. Review. - Development of genetically flexible mouse models of sarcoma using RCAS-TVA mediated gene delivery.
Kabaroff L, Gupta A, Menezes S, Babichev Y, Kandel RC, Swallow CJ, Dickson BC, Gladdy RA. Kabaroff L, et al. PLoS One. 2014 Apr 14;9(4):e94817. doi: 10.1371/journal.pone.0094817. eCollection 2014. PLoS One. 2014. PMID: 24733554 Free PMC article. - CRISPR therapeutic tools for complex genetic disorders and cancer (Review).
Baliou S, Adamaki M, Kyriakopoulos AM, Spandidos DA, Panayiotidis M, Christodoulou I, Zoumpourlis V. Baliou S, et al. Int J Oncol. 2018 Aug;53(2):443-468. doi: 10.3892/ijo.2018.4434. Epub 2018 Jun 6. Int J Oncol. 2018. PMID: 29901119 Free PMC article. Review.
References
- Sharpless NE, Depinho RA. The mighty mouse: genetically engineered mouse models in cancer drug development. Nature Rev Drug Discov. 2006;5:741–754. - PubMed
- Huettner CS, Zhang P, Van Etten RA, Tenen DG. Reversibility of acute B-cell leukaemia induced by BCR-ABL1. Nature Genet. 2000;24:57–60. - PubMed
- Jonkers J, Berns A. Conditional mouse models of sporadic cancer. Nature Rev Cancer. 2002;2:251–265. - PubMed
- Chin L, et al. Essential role for oncogenic Ras in tumour maintenance. Nature. 1999;400:468–472. The first conditional transgenic mouse model to demonstrate solid tumour regression on extinction of the initiating oncogene. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases