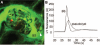A high-powered view of the filtration barrier - PubMed (original) (raw)
A high-powered view of the filtration barrier
János Peti-Peterdi et al. J Am Soc Nephrol. 2010 Nov.
Abstract
Multiphoton excitation fluorescence microscopy is a powerful noninvasive imaging technique for the deep optical sectioning of living tissues. Its application in several intact tissues is a significant advance in our understanding of organ function, including renal pathophysiological mechanisms. The glomerulus, the filtering unit in the kidney, is one good example of a relatively inaccessible and complex structure, with cell types that are otherwise difficult to study at high resolution in their native environment. In this article, we address the application, advantages, and limitations of this imaging technology for the study of the glomerular filtration barrier and the controversy it recently generated regarding the glomerular filtration of macromolecules. More advanced and accurate multiphoton determinations of the glomerular sieving coefficient that are presented here dismiss previous claims on the filtration of nephrotic levels of albumin. The sieving coefficient of 70-kD dextran was found to be around 0.001. Using a model of focal segmental glomerulosclerosis, increased filtration barrier permeability is restricted only to areas of podocyte damage, consistent with the generally accepted role of podocytes and the glomerular origin of albuminuria. Time-lapse imaging provides new details and important in vivo confirmation of the dynamics of podocyte movement, shedding, replacement, and the role of the parietal epithelial cells and Bowman's capsule in the pathology of glomerulosclerosis.
Figures
Figure 1
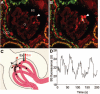
Limitations of the multiphoton fluorescence imaging approach when measuring glomerular sieving of macromolecules. (A) In vivo multiphoton image of an intact glomerulus (G) from a Munich-Wistar-Fromter rat. The intravascular space (plasma) is labeled red using 70-kD dextran-rhodamine B. Red blood cells appear in high density as unlabeled, dark objects in the capillary lumen. A minor, invisible fraction of 70-kD dextran is filtered into the Bowman's space (BS), and it becomes highly concentrated (fluorescence) in the tubular lumen of the cortical collecting duct (CCD). Note the relatively low level of red fluorescence in the glomerular capillary lumen. However, when only red blood cell-free, clear plasma flows in a capillary loop (B, arrowhead, the same glomerulus is shown as in A using the same imaging settings), the plasma fluorescence increases significantly. The flow of red blood cells was obstructed by a small blood clot (arrow), which was formed temporarily in response to briefly focusing the multiphoton laser beam on the luminal surface of one endothelial cell upstream in the glomerular capillary. (C) Illustration of the highly light absorbing and scattering effect of red blood cells streaming in the capillary lumen in high density that results in low efficiency of fluorophore excitation (Ex) and detection of emitted fluorescence (Em). In contrast, the excitation and fluorescence detection are highly efficient in clear solutions (red blood cell-free capillary). (D) Recording of low levels of rhodamine B-fluorescence in the Bowman's space (BS). The level of fluorescence intensity is not steady state; normally, it shows regular oscillations.
Figure 2
Multiphoton images of glomeruli in vivo in (A) control or (B) PAN-treated Munich-Wistar-Fromter rat and (C) control C57BL6 mouse kidneys. The intravascular space (plasma) marker 70-kD dextran-rhodamine B (red) was given in bolus, and Lucifer yellow, a 0.4-kD easily filterable, but cell membrane impermeable, small molecule (green) was infused continuously into the carotid artery to label the primary filtrate in the Bowman's space (BS) green. The vascular endothelium (E) and mesangial cells (M) are intensely labeled green-yellow because they readily endocytose Lucifer yellow as opposed to podocytes (arrowhead, enlarged in inset in A) and parietal cells (arrow) that normally do not and therefore remain unlabeled (dark, negative image). (B) Numerous pseudocysts under the podocytes (asterisk) are clearly visible after PAN treatment.
Figure 3
Podocyte pseudocysts that form as a result of PAN treatment are enlargements of the subpodocyte space. (A) In vivo multiphoton image of an intact glomerulus from a PAN-treated Munich-Wistar-Fromter rat. The intravascular space (plasma) is labeled red using 70-kD dextran-rhodamine. (B) Lucifer yellow was injected into the carotid artery in bolus, and the time-lapse of its fluorescence (green) was recorded in the Bowman's space (BS) and inside pseudocysts (arrowhead) as shown in A. Lucifer yellow appeared and cleared from the BS quickly, whereas the filling and emptying in pseudocysts were slightly slower and delayed.
Figure 4
Increased GFB permeability is restricted to areas of podocyte damage. (A) In vivo multiphoton image of a glomerulus from a PAN-treated Munich-Wistar-Fromter rat. The intravascular space (plasma) is labeled red using 70-kD dextran-rhodamine. (B) Cell nuclei are labeled weakly using Hoechst33342 and a green filter. The time-lapse of rhodamine B fluorescence was recorded in the Bowman's space (BS) as shown in A in two regions of interest: around a damaged, shedding podocyte (arrowhead, ROI1) and around an intact capillary loop (ROI2). GFB permeability to 70-kD dextran was 5- to 10-fold higher around a damaged podocyte. Focal thrombus formation is visible in the capillary loop directly underneath the shedding podocyte.
Figure 5
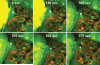
Time-lapse imaging of the shedding and replacement of podocytes in vivo in PAN-treated Munich-Wistar-Fromter rats. The intravascular space (plasma) marker 70-kD dextran-rhodamine B (red) was given in bolus, whereas Lucifer yellow was continuously infused into the carotid artery to label the Bowman's space and visualize podocytes by their lack of labeling (dark cells). Time-series images show the detachment of a pseudocyst-containing podocyte (arrowhead) off of the glomerular capillary wall, its leaving the glomerulus by breaking through the parietal Bowman's capsule, and quick replacement by another newly recruited cell (arrow). After the disappearance of the pseudocyst, the amorphous, detaching podocyte becomes membrane-permeable and highly fluorescence (visible at 500 seconds). After this point, the remaining process is very fast, including complete podocyte detachment and its crossing through the parietal Bowman's capsule within 10 seconds and the appearance of an elongated, stellate-shaped cell (arrow) from the parietal area of the Bowman's capsule within another 10 seconds. Within another 50 seconds, this new cell appears to replace the old, detached podocyte.
Similar articles
- OPN deficiency results in severe glomerulosclerosis in uninephrectomized mice.
Schordan S, Grisk O, Schordan E, Miehe B, Rumpel E, Endlich K, Giebel J, Endlich N. Schordan S, et al. Am J Physiol Renal Physiol. 2013 Jun 15;304(12):F1458-70. doi: 10.1152/ajprenal.00615.2012. Epub 2013 Apr 3. Am J Physiol Renal Physiol. 2013. PMID: 23552865 - Renal albumin handling: a look at the dark side of the filter.
Gekle M. Gekle M. Kidney Int. 2007 Mar;71(6):479-81. doi: 10.1038/sj.ki.5002123. Kidney Int. 2007. PMID: 17344895 - Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat.
Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, Kershaw D, Wiggins R. Kim YH, et al. Kidney Int. 2001 Sep;60(3):957-68. doi: 10.1046/j.1523-1755.2001.060003957.x. Kidney Int. 2001. PMID: 11532090 - The role of the podocyte in glomerulosclerosis.
Kriz W, Lemley KV. Kriz W, et al. Curr Opin Nephrol Hypertens. 1999 Jul;8(4):489-97. doi: 10.1097/00041552-199907000-00014. Curr Opin Nephrol Hypertens. 1999. PMID: 10491745 Review. - In vivo microscopy.
Peti-Peterdi J. Peti-Peterdi J. Nephrol Ther. 2016 Apr;12 Suppl 1(Suppl 1):S21-4. doi: 10.1016/j.nephro.2016.01.004. Epub 2016 Mar 8. Nephrol Ther. 2016. PMID: 26968479 Free PMC article. Review.
Cited by
- Add-on aliskiren elicits stronger renoprotection than high-dose valsartan in type 2 diabetic KKAy mice that do not respond to low-dose valsartan.
Lei B, Nakano D, Fan YY, Kitada K, Hitomi H, Kobori H, Mori H, Masaki T, Nishiyama A. Lei B, et al. J Pharmacol Sci. 2012;119(2):131-8. doi: 10.1254/jphs.12031fp. Epub 2012 May 22. J Pharmacol Sci. 2012. PMID: 22673148 Free PMC article. - Mechanisms of glomerular albumin filtration and tubular reabsorption.
Tojo A, Kinugasa S. Tojo A, et al. Int J Nephrol. 2012;2012:481520. doi: 10.1155/2012/481520. Epub 2012 May 20. Int J Nephrol. 2012. PMID: 22685655 Free PMC article. - Novel fluorescence techniques to quantitate renal cell biology.
Shroff UN, Schiessl IM, Gyarmati G, Riquier-Brison A, Peti-Peterdi J. Shroff UN, et al. Methods Cell Biol. 2019;154:85-107. doi: 10.1016/bs.mcb.2019.04.013. Epub 2019 May 17. Methods Cell Biol. 2019. PMID: 31493823 Free PMC article. - The first decade of using multiphoton microscopy for high-power kidney imaging.
Peti-Peterdi J, Burford JL, Hackl MJ. Peti-Peterdi J, et al. Am J Physiol Renal Physiol. 2012 Jan 15;302(2):F227-33. doi: 10.1152/ajprenal.00561.2011. Epub 2011 Oct 26. Am J Physiol Renal Physiol. 2012. PMID: 22031850 Free PMC article. Review. - Novel in vivo techniques to visualize kidney anatomy and function.
Peti-Peterdi J, Kidokoro K, Riquier-Brison A. Peti-Peterdi J, et al. Kidney Int. 2015 Jul;88(1):44-51. doi: 10.1038/ki.2015.65. Epub 2015 Mar 4. Kidney Int. 2015. PMID: 25738253 Free PMC article. Review.
References
- Denk W, Strickler J, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. - PubMed
- Peti-Peterdi J, Morishima S, Bell PD, Okada Y. Two-photon excitation fluorescence imaging of the living juxtaglomerular apparatus. Am J Physiol Renal Physiol. 2002;283:F197–F201. - PubMed
- Dunn KW, Sandoval RM, Kelly KJ, Dagher PC, Tanner GA, Atkinson SJ, Bacallao RL, Molitoris BA. Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am J Physiol Cell Physiol. 2002;283:C905–C916. - PubMed
- Peti-Peterdi J. Multiphoton imaging of renal tissues in vitro. Am J Physiol Renal Physiol. 2005;288:F1079–F1083. - PubMed
- Molitoris BA, Sandoval RM. Intravital multiphoton microscopy of dynamic renal processes. Am J Physiol Renal Physiol. 2005;288:F1084–F109. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources