A natural BH3 mimetic induces autophagy in apoptosis-resistant prostate cancer via modulating Bcl-2-Beclin1 interaction at endoplasmic reticulum - PubMed (original) (raw)
A natural BH3 mimetic induces autophagy in apoptosis-resistant prostate cancer via modulating Bcl-2-Beclin1 interaction at endoplasmic reticulum
J Lian et al. Cell Death Differ. 2011 Jan.
Abstract
A natural BH3-mimetic, small-molecule inhibitor of Bcl-2, (-)-gossypol, shows promise in ongoing phase II and III clinical trials for human prostate cancer. In this study we show that (-)-gossypol preferentially induces autophagy in androgen-independent (AI) prostate cancer cells that have high levels of Bcl-2 and are resistant to apoptosis, both in vitro and in vivo, but not in androgen-dependent (AD) cells with low Bcl-2 and sensitive to apoptosis. The Bcl-2 inhibitor induces autophagy through blocking Bcl-2-Beclin1 interaction, together with downregulating Bcl-2, upregulating Beclin1, and activating the autophagic pathway. The (-)-gossypol-induced autophagy is dependent on Beclin1 and Atg5. Our results show for the first time that (-)-gossypol can also interrupt the interactions between Beclin1 and Bcl-2/Bcl-xL at endoplasmic reticulum, thus releasing the BH3-only pro-autophagic protein Beclin1, which in turn triggers the autophagic cascade. Oral administration of (-)-gossypol significantly inhibited the growth of AI prostate cancer xenografts, representing a promising new regimen for the treatment of human hormone-refractory prostate cancer with Bcl-2 overexpression. Our data provide new insights into the mode of cell death induced by Bcl-2 inhibitors, which will facilitate the rational design of clinical trials by selecting patients who are most likely to benefit from the Bcl-2-targeted molecular therapy.
Figures
Figure 1
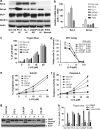
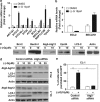
(−)-Gossypol preferentially induces apoptosis in human prostate cancer cells with low Bcl-2, but is equally potent in inducing non-apoptotic cell death in cells with high levels of Bcl-2. (a) Western blot analysis of the protein levels of Bcl-2, Bcl-xL, Mcl-1, and Beclin1 in prostate cancer cell lines and normal prostate epithelial cells (PrECs); (b) mRNA levels of Bcl-2 and Bcl-xL in these cells by qRT-PCR. (c) (−)-Gossypol dose-dependently induces cells death in prostate cancer cells regardless of their Bcl-2 levels. Cells (1 × 104) were seeded in a 12-well plate overnight, and then treated with different doses of (−)-gossypol. After 24 h, they were trypsinized and counted after Trypan blue staining. Data are presented as percentage of dead cells. Results are mean±S.D. of three independent experiments. (d) MTT-based cytotoxicity assay of (−)-gossypol in prostate cancer cells. Cells were seeded in 96-well plates and treated in triplicates. (e) (−)-Gossypol-induced apoptosis in prostate cancer cells as assayed by sub-G1 analysis. After being treated with (−)-gossypol for 24 h, the cells were fixed by ethanol, stained with PI, and analyzed by flow cytometry. (f) (−)-Gossypol-induced caspase-3 activation in prostate cancer cell lines. Cells (1 × 105) were seeded in a 12-well plate overnight and then treated with (−)-gossypol. After 24 h, caspase-3 activity was measured. (g) PARP cleavage in prostate cancer cells treated with 10 _μ_M (−)-gossypol for 24 h. (h) After overnight culture, cells were treated with DMSO, 10 _μ_M (−)-gossypol, or (−)-gossypol ((−)-G) combined with Z-VAD (10 _μ_M) for 24 h. Cells were then trypsinized and counted after Trypan blue staining. Results are means±S.D. of three independent experiments. **P<0.01 between (−)-G and (−)-G+Z-VAD in indicated cell lines by two-way ANOVA
Figure 2
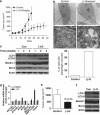
(−)-Gossypol preferentially induces autophagy in apoptosis-resistant prostate cancer cells with high levels of Bcl-2 but not in cells with low Bcl-2. (a) (−)-Gossypol-induced autophagy in prostate cancer cells as revealed by LC3-II conversion in western blot analysis. Cells were treated with DMSO or 10 _μ_M (−)-gossypol for 24 h, and then lysed for western blot of LC3. 3-MA 5 mM and rapamycin 0.5 _μ_M were used as an inhibitor and an inducer of autophagy, respectively. (b, c) Dose response (b) and time course (c) of (−)-gossypol-induced autophagy in PC-3 and CL-1 cells. (d) Representative electron microscopic images showing autophagic vacuoles with content (black arrows) after (−)-gossypol treatment. The percentage of cells with autophagic vacuoles was quantified in 50 cells each group
Figure 3
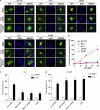
(−)-Gossypol preferentially induces autophagy in apoptosis-resistant prostate cancer cells as revealed by LC3-GFP puncta formation. (a) (−)-Gossypol-induced autophagy in prostate cancer cells as analyzed by LC3-GFP. Cells were transfected with LC3-GFP plasmid, treated with DMSO or 10 _μ_M (−)-gossypol for 24 h, and then analyzed under a fluorescent microscope. The yellow arrows indicate the punctate pattern of LC3-GFP in autophagic cells. Treatment with 0.5 _μ_M rapamycin was used as a positive control for autophagy induction. (b) Quantification of data from (a), expressed as percentage of cells with punctate LC3-GFP (50 green fluorescent cells in one field, _n_=5). (c, d) Effects of Atg5 or Beclin1 downregulation or 3-MA on the (−)-gossypol-induced cell death in CL-1(c) and LNCaP cells (d). Cells were transiently transfected either with a control siRNA or siRNA specific to Atg5 or Beclin1 or pre-treated with 5 mM 3-MA, and then treated with DMSO or (−)-gossypol for 24 h. Viability was assessed by Trypan blue staining. Results are means±S.D. of three independent experiments. **P<0.01 or *P<0.05 by two-way ANOVA compared to (−)-G treatment of con-siRNA cells where indicated in panel (c) and (d), respectively
Figure 4
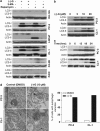
(−)-Gossypol modulates Bcl-2–Beclin1 interaction at the ER. Co-immunoprecipitation (co-IP) pull-down assay shows that (−)-gossypol specifically disrupts Bcl-2–Beclin1 interaction. CL-1 cells were treated with DMSO or 10 _μ_M (−)-gossypol for 6 h at 37°C and subjected to subcellular fractionation and IP with the indicated antibodies
Figure 5
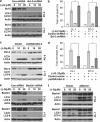
Effects of modulating Bcl-2 or Beclin1 protein levels on (−)-gossypol-induced autophagy and cell death in PC-3 and CL-1 cells. (a–f) Cells were transiently transfected with either control shRNA/siRNA or shRNA/siRNA specific to Bcl-2 (a)/Beclin1 (e), or expression vectors for Bcl-2 (b) or Beclin1 (f). At 24 h after transfection, cells were treated with DMSO or (−)-gossypol for 24 h, and then subjected to either immunoblot analysis for Bcl-2, Beclin1, and LC3 or Trypan blue staining (b, d). *P<0.05 between the indicated groups by two-way ANOVA in panels (b) and (d)
Figure 6
(−)-Gossypol regulates autophagy pathway-associated genes in prostate cancer cells. (a) Human Autophagy PCR Array analysis of the autophagy-associated gene expression levels in CL-1 cells treated with DMSO or 10 _μ_M (−)-gossypol. (_n_=2). (b) qRT-PCR validation of Bcl-2 and Beclin1 expression. (c) Western blot confirmation of the protein-level changes of the most regulated genes identified by PCR Array. (d) PC-3 and CL-1 cells were transiently transfected with either control siRNA or SmartPool Atg5-siRNAs for 24 h, and then treated with DMSO or (−)-gossypol for 24 h. The cells were lysed for immunoblot analysis of Atg5-12 and LC3. (e) Percentage of cells with punctate GFP-LC3 in CL-1 cells transiently transfected with control siRNA or Atg5-siRNA treated with DMSO (control) or 10 _μ_M (−)-gossypol for 24 h. **P<0.01 between the indicated groups by two-way ANOVA
Figure 7
Oral administration of (−)-gossypol inhibited CL-1 and PC-3 xenograft growth and was associated with increased LC3-II conversion in the tumors. (a) (−)-gossypol potently inhibited the CL-1 xenograft tumor growth in nude mice as a single-agent oral therapy. CL-1 cells (2 × 106) were s.c. injected into the flanks on both sides of each mouse. When the tumors reached 100 mm3, the mice were randomized into 5–8 mice per group. (−)-Gossypol was administrated p.o. through oral gavage, daily at a 20 mg/kg dose. The data shown are average tumor size (means±S.E.M., _n_=10). **P<0.01, versus vehicle control, two-way ANOVA (_n_=10). (b) Representative electron micrograph image showing autophagic vacuoles with content after (−)-gossypol treatment in an in vivo CL-1 xenograft model. The percentage of cells with autophagic vacuoles was quantified in 50 cells per group. (c) (−)-Gossypol-induced autophagy in CL-1 xenograft tumors in vivo. CL-1 xenograft tumor tissue lysates from the vehicle control group or (−)-gossypol treatment group were immunoblotted for Beclin1, LC3, and Bcl-2 at the indicated time points. (d) Modulation of the autophagy-associated gene expression in tumor tissues after treatment with DMSO or (−)-gossypol for 2 weeks. Gene expression was detected using Human Autophagy PCR Array and the data are shown as relative mRNA levels (_n_=2). (e) (−)-Gossypol potently inhibited the PC-3 xenograft tumor growth in nude mice as a single-agent oral therapy. Study was conducted as in (a) and tumor size data were collected at 5 weeks. (f) (−)-Gossypol-induced autophagy in vivo in PC-3 xenograft model in nude mice. Immunoblotting for Beclin1, LC3, and Bcl-2 using lysates from PC-3 xenograft tumor tissues treated with vehicle or (−)-gossypol for 3 weeks. *P<0.05 by two-way ANOVA
Figure 8
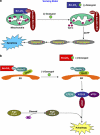
Model for the mechanisms of action of (−)-gossypol, indicating that the mode of cell death induced by (−)-gossypol is cellular context dependent. (a) In androgen-dependent (AD) prostate cancer cells that have low levels of Bcl-2/xL and are sensitive to apoptosis, for example, LNCaP, (−)-gossypol potently binds to Bcl-2/xL at mitochondria, releasing Bax/Bak and inducing apoptotic cell death. (b) In androgen-independent (AI) prostate cancer cells that have high levels of Bcl-2/xL and are resistant to apoptosis, for example, CL-1 and PC-3, (−)-gossypol potently binds to Bcl-2/xL and releases Beclin1 at ER, and thus preferentially induces autophagic cell death
Similar articles
- The Bcl-2-Beclin 1 interaction in (-)-gossypol-induced autophagy versus apoptosis in prostate cancer cells.
Lian J, Karnak D, Xu L. Lian J, et al. Autophagy. 2010 Nov;6(8):1201-3. doi: 10.1038/cdd.2010.74. Epub 2010 Nov 16. Autophagy. 2010. PMID: 20930561 Free PMC article. - Natural BH3 mimetic (-)-gossypol chemosensitizes human prostate cancer via Bcl-xL inhibition accompanied by increase of Puma and Noxa.
Meng Y, Tang W, Dai Y, Wu X, Liu M, Ji Q, Ji M, Pienta K, Lawrence T, Xu L. Meng Y, et al. Mol Cancer Ther. 2008 Jul;7(7):2192-202. doi: 10.1158/1535-7163.MCT-08-0333. Mol Cancer Ther. 2008. PMID: 18645028 Free PMC article. - The Bcl-2 homology domain 3 mimetic gossypol induces both Beclin 1-dependent and Beclin 1-independent cytoprotective autophagy in cancer cells.
Gao P, Bauvy C, Souquère S, Tonelli G, Liu L, Zhu Y, Qiao Z, Bakula D, Proikas-Cezanne T, Pierron G, Codogno P, Chen Q, Mehrpour M. Gao P, et al. J Biol Chem. 2010 Aug 13;285(33):25570-81. doi: 10.1074/jbc.M110.118125. Epub 2010 Jun 7. J Biol Chem. 2010. PMID: 20529838 Free PMC article. - Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.
Salminen A, Kaarniranta K, Kauppinen A, Ojala J, Haapasalo A, Soininen H, Hiltunen M. Salminen A, et al. Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review. - Autophagy contributes to modulating the cytotoxicities of Bcl-2 homology domain-3 mimetics.
Yu L, Liu S. Yu L, et al. Semin Cancer Biol. 2013 Dec;23(6 Pt B):553-60. doi: 10.1016/j.semcancer.2013.08.008. Epub 2013 Sep 4. Semin Cancer Biol. 2013. PMID: 24012660 Review.
Cited by
- Cadmium toxicity and autophagy: a review.
Shao Y, Zheng L, Jiang Y. Shao Y, et al. Biometals. 2024 Jun;37(3):609-629. doi: 10.1007/s10534-023-00581-y. Epub 2024 Jan 26. Biometals. 2024. PMID: 38277035 Review. - Knockdown of BAG3 sensitizes bladder cancer cells to treatment with the BH3 mimetic ABT-737.
Mani J, Antonietti P, Rakel S, Blaheta R, Bartsch G, Haferkamp A, Kögel D. Mani J, et al. World J Urol. 2016 Feb;34(2):197-205. doi: 10.1007/s00345-015-1616-2. Epub 2015 Jun 23. World J Urol. 2016. PMID: 26100943 - Roles of autophagy in androgen-induced benign prostatic hyperplasia in castrated rats.
Liu RF, Fu G, Li J, Yang YF, Wang XG, Bai PD, Chen YD. Liu RF, et al. Exp Ther Med. 2018 Mar;15(3):2703-2710. doi: 10.3892/etm.2018.5772. Epub 2018 Jan 19. Exp Ther Med. 2018. PMID: 29456672 Free PMC article. - Natural proteasome inhibitor celastrol suppresses androgen-independent prostate cancer progression by modulating apoptotic proteins and NF-kappaB.
Dai Y, Desano J, Tang W, Meng X, Meng Y, Burstein E, Lawrence TS, Xu L. Dai Y, et al. PLoS One. 2010 Dec 10;5(12):e14153. doi: 10.1371/journal.pone.0014153. PLoS One. 2010. PMID: 21170316 Free PMC article. - Proteomic analysis of gossypol induces necrosis in multiple myeloma cells.
Xu R, Tian E, Tang H, Liu C, Wang Q. Xu R, et al. Biomed Res Int. 2014;2014:839232. doi: 10.1155/2014/839232. Epub 2014 Aug 14. Biomed Res Int. 2014. PMID: 25197664 Free PMC article.
References
- Assikis VJ, Simons JW. Novel therapeutic strategies for androgen-independent prostate cancer: an update. Semin Oncol. 2004;31 (2 Suppl 4:26–32. - PubMed
- Oh WK, Kantoff PW. Management of hormone refractory prostate cancer: current standards and future prospects.[see comment]. [Review] [107 refs] J Urol. 1998;160:1220–1229. - PubMed
- Rago R. Management of hormone-sensitive and hormone-refractory metastatic prostate cancer. Cancer Control. 1998;5:513–521. - PubMed
- Lin J, Zheng Z, Li Y, Yu W, Zhong W, Tian S, et al. A novel Bcl-XL inhibitor Z36 that induces autophagic cell death in Hela cells. Autophagy. 2009;5:314–320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA121830-04S1/CA/NCI NIH HHS/United States
- R01 CA121830/CA/NCI NIH HHS/United States
- P30 CA046592/CA/NCI NIH HHS/United States
- P30 CA46592/CA/NCI NIH HHS/United States
- R21 CA128220-02/CA/NCI NIH HHS/United States
- CA121830/CA/NCI NIH HHS/United States
- R01 CA121830-04/CA/NCI NIH HHS/United States
- R01 CA134655/CA/NCI NIH HHS/United States
- CA128220/CA/NCI NIH HHS/United States
- CA134655/CA/NCI NIH HHS/United States
- R21 CA128220/CA/NCI NIH HHS/United States
- R01 CA134655-01A1/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials