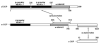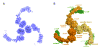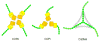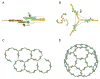Structure of coatomer cage proteins and the relationship among COPI, COPII, and clathrin vesicle coats - PubMed (original) (raw)
Structure of coatomer cage proteins and the relationship among COPI, COPII, and clathrin vesicle coats
Changwook Lee et al. Cell. 2010.
Abstract
COPI-coated vesicles form at the Golgi apparatus from two cytosolic components, ARF G protein and coatomer, a heptameric complex that can polymerize into a cage to deform the membrane into a bud. Although coatomer shares a common evolutionary origin with COPII and clathrin vesicle coat proteins, the architectural relationship among the three cages is unclear. Strikingly, the alphabeta'-COP core of coatomer crystallizes as a triskelion in which three copies of a beta'-COP beta-propeller domain converge through their axial ends. We infer that the trimer constitutes the vertex of the COPI cage. Our model proposes that the COPI cage is intermediate in design between COPII and clathrin: COPI shares with clathrin an arrangement of three curved alpha-solenoid legs radiating from a common center, and COPI shares with COPII highly similar vertex interactions involving the axial ends of beta-propeller domains.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
Figure 1. Domain Organization of the αβ′ε-COP Complex
Diagram shows the domain structure of B. taurus αβ′ε-COP, as defined by proteolytic mapping and sequence/structure analysis. The products of limit proteolysis reactions were separated chromatographically to identify discrete subcomplexes and domains (see Experimental Procedures). The two subcomplexes for which crystal structures were determined—αβ′-COP and αε-COP—are indicated with arrows. Note that we determined the crystal structure of the B. taurus αε-COP subcomplex, and the S.cerevisiae αβ′-COP subcomplex comprising β′-COP residues 1-814 (equivalent to B. taurus residues 1-798) and α-COP residues 642-818 (equivalent to B. taurus residues 638-813).
Figure 2. Structural Analysis of the αβ′-COP Subcomplex
(A) Experimental electron density map (calculated with data to 3.1 Å resolution and contoured at 1.5 σ) of the crystal asymmetric unit of the αβ′-COP subcomplex. This map was calculated with the MIRAS phases following density modification including three-fold non-crystallographic symmetry (NCS) averaging. The map is viewed along the three-fold NCS axis. (B) Surface representation of the αβ′-COP triskelion. The figure is oriented as in (A), with the three β′-COP subunits colored in three shades of orange, and with the α-COP subunits colored three shades of green. The label “β-propeller domains” denotes to the two β-propeller domains on each copy of β′-COP.
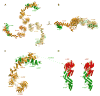
Figure 3. Architecture of the αβ′-COP and αε-COP Subcomplexes
(A) Ribbon diagram of the αβ′-COP triskelion, viewed in the same orientation as Figure 2. Three copies of the αβ′-COP heterodimer associate in the crystal asymmetric unit. We infer that this arrangement corresponds to the vertex of the COPI cage. The crystal structure was determined by MIRAS phasing and refined to 2.5 Å resolution (Table 1). The complex comprises residues 1-814 of β′-COP and residues 642-818 of α-COP (colored as in Figure 2B). β strands are drawn as arrows and α helices as cylinders. (B) This view of the αβ′-COP triskelion is rotated 90° about a horizontal axis relative to (A). (C) Close-up view of one copy of the αβ′-COP subcomplex, in the same orientation as the uppermost copy in (A). Starting from its N terminus, the β′-COP subunit (orange) has two β-propeller domains followed by an α-solenoid domain comprising fifteen α helices. In the structure, the α-COP subunit (green) has twelve α helices; these are numbered from helix α4 to highlight the similarity to the solenoid domain of β′-COP (see text for details). See also Figures S1, S3 and S4. (D) Ribbon diagram of the αε-COP subcomplex drawn as a stereo pair. This structure was determined by MAD phasing and refined to 2.6 Å resolution (Table 1). In the picture α-COP is colored green and ε-COP is red.
Figure 4. Contact surfaces of the αβ′-COP triskelion
(A) The picture on the left shows the αβ′-COP trimer viewed along the three-fold symmetry axis. The close-up view on the right shows the residues of β′-COP that contribute to the triskelion contact surfaces. The three copies of residue Ser114 are drawn as CPK spheres and their locations indicated by asterisks; this residue is mutated to tyrosine in the sec27-95 mutant (Eugster et al., 2004). Oxygen and nitrogen atoms are colored red and blue, respectively. (B) In this picture, the top left copy of αβ′-COP from (A) has been rotated 90° about a vertical axis. The view is along the axes of the β-propeller domains of β′-COP. The seven blades of the N-terminal β-propeller domain are labeled 1–7; and the four β strands of blade 1 are labeled A–D (this nomenclature is used in the sequence alignment Figure S1). (C) Surface representation of the αβ′-COP molecule in (B). Residues on the N-terminal β-propeller involved in interfacial contacts at the triskelion center are colored red. Two yeast mutants that were isolated in genetic screens, sec27-1 and sec27-95, harbor mutations in key regions of the β′-COP molecule: the sec27-1 mutation G688D is located beneath the protein surface near the α-solenoid/α-solenoid interface with α-COP; the sec27-95 mutation S114Y is located just beneath surface residues that form contacts at the triskelion center.
Figure 5. Relationship among COPI, COPII and clathrin cages
Schematic diagram compares the vertex geometry of COPI, COPII and clathrin cages. The inner β-propeller domains that form vertex contacts are drawn as orange cylinders, the outer β-propeller domains as yellow cylinders, and the α-solenoid domains as linked green hexagons. (The hexagons are purely schematic; their size and number have no meaning). See also Figure S2.
Figure 6. Model for the architecture of the COPI lattice
(A) Known and unknown elements of the αβ′ε-COP complex. The αβ′-COP and αε-COP subcomplexes (circled with dotted lines) whose structures we have determined account for more than two thirds of the total mass of the αβ′ε-COP complex. The remaining portion is the N-terminal region of α-COP whose sequence indicates an N-terminal β-propeller domain followed by a ∼300-residue region of unknown structure (possibly a second β-propeller domain). The speculative element of the diagram is the dimer contact (indicated by the question mark) that brings together two copies of αβ′ε-COP. We have drawn this as an α-COP–α-COP interaction as one possibility; alternatively β′-COP might mediate this interaction. Either way, we propose that two copies of αβ′ε-COP would converge to form the assembly unit COPI. (B) A model for the COPI vertex, and corresponding ribbon diagrams of the αβ′-COP and αε-COP crystal structures. (C) Based on a three-fold rotation axis at the vertex, the COPI cage symmetry most likely is related to clathrin cage symmetry. Thus the COPI lattice model is composed of hexagons and pentagons. (D) One possible arrangement of hexagonal and pentagonal units forming an icosahedral COPI cage. This type of structure is formed by clathrin in vitro (Fotin et al., 2004).
Comment in
- Copy coats: COPI mimics clathrin and COPII.
Hughson FM. Hughson FM. Cell. 2010 Jul 9;142(1):19-21. doi: 10.1016/j.cell.2010.06.031. Cell. 2010. PMID: 20603010
Similar articles
- The structures of COPI-coated vesicles reveal alternate coatomer conformations and interactions.
Faini M, Prinz S, Beck R, Schorb M, Riches JD, Bacia K, Brügger B, Wieland FT, Briggs JA. Faini M, et al. Science. 2012 Jun 15;336(6087):1451-4. doi: 10.1126/science.1221443. Epub 2012 May 24. Science. 2012. PMID: 22628556 - Crystal structure of alpha-COP in complex with epsilon-COP provides insight into the architecture of the COPI vesicular coat.
Hsia KC, Hoelz A. Hsia KC, et al. Proc Natl Acad Sci U S A. 2010 Jun 22;107(25):11271-6. doi: 10.1073/pnas.1006297107. Epub 2010 Jun 3. Proc Natl Acad Sci U S A. 2010. PMID: 20534429 Free PMC article. - Structure and organization of coat proteins in the COPII cage.
Fath S, Mancias JD, Bi X, Goldberg J. Fath S, et al. Cell. 2007 Jun 29;129(7):1325-36. doi: 10.1016/j.cell.2007.05.036. Cell. 2007. PMID: 17604721 - Structure and mechanism of COPI vesicle biogenesis.
Jackson LP. Jackson LP. Curr Opin Cell Biol. 2014 Aug;29:67-73. doi: 10.1016/j.ceb.2014.04.009. Epub 2014 May 17. Curr Opin Cell Biol. 2014. PMID: 24840894 Review. - Vesicle coats: structure, function, and general principles of assembly.
Faini M, Beck R, Wieland FT, Briggs JA. Faini M, et al. Trends Cell Biol. 2013 Jun;23(6):279-88. doi: 10.1016/j.tcb.2013.01.005. Epub 2013 Feb 13. Trends Cell Biol. 2013. PMID: 23414967 Review.
Cited by
- Structure, dynamics, evolution, and function of a major scaffold component in the nuclear pore complex.
Sampathkumar P, Kim SJ, Upla P, Rice WJ, Phillips J, Timney BL, Pieper U, Bonanno JB, Fernandez-Martinez J, Hakhverdyan Z, Ketaren NE, Matsui T, Weiss TM, Stokes DL, Sauder JM, Burley SK, Sali A, Rout MP, Almo SC. Sampathkumar P, et al. Structure. 2013 Apr 2;21(4):560-71. doi: 10.1016/j.str.2013.02.005. Epub 2013 Mar 14. Structure. 2013. PMID: 23499021 Free PMC article. - Evolution: On a bender--BARs, ESCRTs, COPs, and finally getting your coat.
Field MC, Sali A, Rout MP. Field MC, et al. J Cell Biol. 2011 Jun 13;193(6):963-72. doi: 10.1083/jcb.201102042. J Cell Biol. 2011. PMID: 21670211 Free PMC article. Review. - Evidence for the involvement of the Arabidopsis SEC24A in male transmission.
Conger R, Chen Y, Fornaciari S, Faso C, Held MA, Renna L, Brandizzi F. Conger R, et al. J Exp Bot. 2011 Oct;62(14):4917-26. doi: 10.1093/jxb/err174. Epub 2011 Jun 24. J Exp Bot. 2011. PMID: 21705385 Free PMC article. - Golgi bypass: skirting around the heart of classical secretion.
Grieve AG, Rabouille C. Grieve AG, et al. Cold Spring Harb Perspect Biol. 2011 Apr 1;3(4):a005298. doi: 10.1101/cshperspect.a005298. Cold Spring Harb Perspect Biol. 2011. PMID: 21441587 Free PMC article. Review. - Molecular basis for recognition of dilysine trafficking motifs by COPI.
Jackson LP, Lewis M, Kent HM, Edeling MA, Evans PR, Duden R, Owen DJ. Jackson LP, et al. Dev Cell. 2012 Dec 11;23(6):1255-62. doi: 10.1016/j.devcel.2012.10.017. Epub 2012 Nov 21. Dev Cell. 2012. PMID: 23177648 Free PMC article.
References
- Antonny B, Beraud-Dufour S, Chardin P, Chabre M. N-terminal hydrophobic residues of the G-protein ADP-ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry. 1997;36:4675–4684. - PubMed
- Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. - PubMed
- Bremser M, Nickel W, Schweikert M, Ravazzola M, Amherdt M, Hughes CA, Sollner TH, Rothman JE, Wieland FT. Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell. 1999;96:495–506. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases