Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series - PubMed (original) (raw)
Comparative Study
Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series
Meizan Lai et al. Lancet Neurol. 2010 Aug.
Abstract
Background: Voltage-gated potassium channels are thought to be the target of antibodies associated with limbic encephalitis. However, antibody testing using cells expressing voltage-gated potassium channels is negative; hence, we aimed to identify the real autoantigen associated with limbic encephalitis.
Methods: We analysed sera and CSF of 57 patients with limbic encephalitis and antibodies attributed to voltage-gated potassium channels and 148 control individuals who had other disorders with or without antibodies against voltage-gated potassium channels. Immunohistochemistry, immunoprecipitation, and mass spectrometry were used to characterise the antigen. An assay with HEK293 cells transfected with leucine-rich, glioma-inactivated 1 (LGI1) and disintegrin and metalloproteinase domain-containing protein 22 (ADAM22) or ADAM23 was used as a serological test. The identity of the autoantigen was confirmed by immunoabsorption studies and immunostaining of Lgi1-null mice.
Findings: Immunoprecipitation and mass spectrometry analyses showed that antibodies from patients with limbic encephalitis previously attributed to voltage-gated potassium channels recognise LGI1, a neuronal secreted protein that interacts with presynaptic ADAM23 and postsynaptic ADAM22. Immunostaining of HEK293 cells transfected with LGI1 showed that sera or CSF from patients, but not those from control individuals, recognised LGI1. Co-transfection of LGI1 with its receptors, ADAM22 or ADAM23, changed the pattern of reactivity and improved detection. LGI1 was confirmed as the autoantigen by specific abrogation of reactivity of sera and CSF from patients after immunoabsorption with LGI1-expressing cells and by comparative immunostaining of wild-type and Lgi1-null mice, which showed selective lack of reactivity in brains of Lgi1-null mice. One patient with limbic encephalitis and antibodies against LGI1 also had antibodies against CASPR2, an autoantigen we identified in some patients with encephalitis and seizures, Morvan's syndrome, and neuromyotonia.
Interpretation: LGI1 is the autoantigen associated with limbic encephalitis previously attributed to voltage-gated potassium channels. The term limbic encephalitis associated with antibodies against voltage-gated potassium channels should be changed to limbic encephalitis associated with LGI1 antibodies, and this disorder should be classed as an autoimmune synaptic encephalopathy.
Funding: National Institutes of Health, National Cancer Institute, and Euroimmun.
Copyright 2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts of interest
A patent application for the use of LGI1 antibody determination in patients’ sera and CSF as a diagnostic test has been filed in the USA by JD. None of the other authors have any conflicts of interest.
Figures
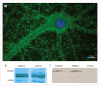
Figure 1. Immunocytochemistry and immunoprecipitation of LGI1 with sera from patients with limbic encephalitis previously attributed to voltage-gated potassium channels
(A) Immunolabelling of a rat hippocampal neuron with serum of a patient (patient 1) with antibodies previously attributed to voltage-gated potassium channels. The nucleus of the neuron is visualised with DAPI. (B) Immunoprecipitates obtained using serum from a patient and a control individual were separated by gel electrophoresis and the gel stained with coomassie blue. A band of about 60 kDa was detected in the sample from the patient and, by mass spectrometry, was identified as LGI1. This band was not present in the sample from the control individual. The protein bands at 55 kDa and 52 kDa correspond to fragments of human IgG. (C) Immunoblot of the precipitates obtained with the sera from patient 1, another patient (patient 2), and a control individual (control). LGI1 was present in the neuronal immunoprecipitates obtained using sera from both patients but not the control individual. The antibody used in this analysis was a polyclonal LGI1 antibody that is commercially available.
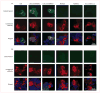
Figure 2. Reactivity of a patient’s serum with HEK293 cells expressing LGI1 alone or coexpressing its receptors, ADAM22 or ADAM23
Patient’s antibodies were detected with a secondary antibody labelled with Alexa Fluor 488 (in green). Antibodies specific for the indicated protein were detected with a secondary antibody labelled with Alexa Fluor 594 (in red). Nuclei were stained with DAPI. For clarity, nuclei staining (blue) is only shown in the merged images. (A) HEK293 cells expressing proteins indicated at the top of the panels immunostained with serum from a patient (top row) and commercial antibodies against each protein (second row). Merged reactivities are shown in the bottom row. When LGI1 is expressed alone, the reactivity with the patient’s antibodies is usually clustered in the cytoplasm, without uniform distribution on the cell surface, and showing partial co-localisation with the reactivity of a commercial antibody. When LGI1 is coexpressed with ADAM22 or ADAM23 (columns 2 and 3), the epitopes targeted by the patient’s antibodies might be better exposed on the cell membrane and co-localisation improves. By contrast, the patient’s antibodies did not bind ADAM22 or ADAM23 when expressed alone, nor did they react with Kv1.1 and Kv1.4 subunits. (B) HEK293 cells expressing proteins as before but incubated with serum from a control individual (top row). Serum from the control individual did not react with any of the proteins.
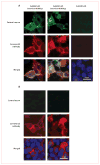
Figure 3. A patient’s serum reacts with soluble LGI1 bound to ADAM22 or ADAM23
Patient’s antibodies were detected with a secondary antibody labelled with Alexa Fluor 488 (in green). Antibodies specific for the indicated protein were detected with a secondary antibody labelled with Alexa Fluor 594 (in red). Nuclei were stained with DAPI. For clarity, nuclei staining (blue) is only shown in the merged images. LGI1-containing media was applied to HEK293 cells expressing ADAM22 (first column) or ADAM23 (second column). (A) Patient’s antibodies recognised LGI1 bound to ADAM22 or ADAM23, but not HEK293 cells without either receptor (third row). (B) Absence of reactivity of serum from a control individual.
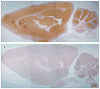
Figure 4. Immunoabsorption confirms that LGI1 is the autoantigen in patients with limbic encephalitis and antibodies attributed to voltage-gated potassium channels
A rat brain section immunostained with serum from a patient with limbic encephalitis and antibodies attributed to voltage-gated potassium channels before (A) and after (B) immunoabsorption with LGI1. All the patients’ serum reactivity (A, brown staining) disappeared in B, indicating that all the patients’ antibodies are directed against LGI1.
Figure 5. Reactivity of patients’ antibodies in wild-type mouse brain is abrogated in _Lgi3_-null mice
Sections of brain of wild-type (A–D) and _Lgi3_-null mice (E–H) incubated with serum from four patients with limbic encephalitis and antibodies attributed to voltage-gated potassium channels. The difference of reactivity between the anterior and posterior cerebellar cortex in the wild-type mice is due to a fixation artifact. Note that the reactivity of patients’ sera (brown staining in A–D) is abrogated in the _Lgi3_-null mice (E–H), indicating that patients’ antibodies are specifically directed against LGI1.
Comment in
- Is autoimmune limbic encephalitis a channelopathy?
Honnorat J. Honnorat J. Lancet Neurol. 2010 Aug;9(8):753-5. doi: 10.1016/S1474-4422(10)70162-9. Epub 2010 Jun 26. Lancet Neurol. 2010. PMID: 20580614 No abstract available. - Neuroimmunology: Antibodies target LGI1 rather than potassium channels in limbic encephalitis.
Yates D. Yates D. Nat Rev Neurol. 2010 Sep;6(9):467. doi: 10.1038/nrneurol.2010.119. Nat Rev Neurol. 2010. PMID: 20836192 No abstract available.
Similar articles
- LGI1 antibodies alter Kv1.1 and AMPA receptors changing synaptic excitability, plasticity and memory.
Petit-Pedrol M, Sell J, Planagumà J, Mannara F, Radosevic M, Haselmann H, Ceanga M, Sabater L, Spatola M, Soto D, Gasull X, Dalmau J, Geis C. Petit-Pedrol M, et al. Brain. 2018 Nov 1;141(11):3144-3159. doi: 10.1093/brain/awy253. Brain. 2018. PMID: 30346486 Free PMC article. - Clinical spectrum and diagnostic value of antibodies against the potassium channel related protein complex.
Montojo MT, Petit-Pedrol M, Graus F, Dalmau J. Montojo MT, et al. Neurologia. 2015 Jun;30(5):295-301. doi: 10.1016/j.nrl.2013.12.007. Epub 2014 Jan 30. Neurologia. 2015. PMID: 24485651 Free PMC article. Review. - [Current Perspective on Voltage-gated Potassium Channel Complex Antibody Associated Diseases].
Watanabe O. Watanabe O. Brain Nerve. 2018 Apr;70(4):315-328. doi: 10.11477/mf.1416201005. Brain Nerve. 2018. PMID: 29632280 Japanese. - Autoantibodies to epilepsy-related LGI1 in limbic encephalitis neutralize LGI1-ADAM22 interaction and reduce synaptic AMPA receptors.
Ohkawa T, Fukata Y, Yamasaki M, Miyazaki T, Yokoi N, Takashima H, Watanabe M, Watanabe O, Fukata M. Ohkawa T, et al. J Neurosci. 2013 Nov 13;33(46):18161-74. doi: 10.1523/JNEUROSCI.3506-13.2013. J Neurosci. 2013. PMID: 24227725 Free PMC article. - Continuous muscle activity, Morvan's syndrome and limbic encephalitis: ionic or non ionic disorders?
Serratrice G, Serratrice J. Serratrice G, et al. Acta Myol. 2011 Jun;30(1):32-3. Acta Myol. 2011. PMID: 21842591 Free PMC article. Review.
Cited by
- Sleep Disturbances Associated with Neurological Autoimmunity.
Devine MF, St Louis EK. Devine MF, et al. Neurotherapeutics. 2021 Jan;18(1):181-201. doi: 10.1007/s13311-021-01020-x. Epub 2021 Mar 30. Neurotherapeutics. 2021. PMID: 33786802 Free PMC article. Review. - Anti-GAD65 Containing Cerebrospinal Fluid Does not Alter GABAergic Transmission.
Hackert JK, Müller L, Rohde M, Bien CG, Köhling R, Kirschstein T. Hackert JK, et al. Front Cell Neurosci. 2016 May 18;10:130. doi: 10.3389/fncel.2016.00130. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27242441 Free PMC article. - Identification of genetic variants of LGI1 and RTN4R (NgR1) linked to schizophrenia that are defective in NgR1-LGI1 signaling.
Thomas RA, Ambalavanan A, Rouleau GA, Barker PA. Thomas RA, et al. Mol Genet Genomic Med. 2016 Mar 11;4(4):447-56. doi: 10.1002/mgg3.215. eCollection 2016 Jul. Mol Genet Genomic Med. 2016. PMID: 27468420 Free PMC article. - Cytotoxic CD8+ T cells and CD138+ plasma cells prevail in cerebrospinal fluid in non-paraneoplastic cerebellar ataxia with contactin-associated protein-2 antibodies.
Melzer N, Golombeck KS, Gross CC, Meuth SG, Wiendl H. Melzer N, et al. J Neuroinflammation. 2012 Jul 3;9:160. doi: 10.1186/1742-2094-9-160. J Neuroinflammation. 2012. PMID: 22759321 Free PMC article. - The LGI1-ADAM22 protein complex directs synapse maturation through regulation of PSD-95 function.
Lovero KL, Fukata Y, Granger AJ, Fukata M, Nicoll RA. Lovero KL, et al. Proc Natl Acad Sci U S A. 2015 Jul 28;112(30):E4129-37. doi: 10.1073/pnas.1511910112. Epub 2015 Jul 15. Proc Natl Acad Sci U S A. 2015. PMID: 26178195 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- RC1 NS068204/NS/NINDS NIH HHS/United States
- NS046706/NS/NINDS NIH HHS/United States
- R01 NS046706/NS/NINDS NIH HHS/United States
- R01CA107192/CA/NCI NIH HHS/United States
- R01 NS077851/NS/NINDS NIH HHS/United States
- R01 CA107192/CA/NCI NIH HHS/United States
- R01 CA089054-02/CA/NCI NIH HHS/United States
- R01 CA089054/CA/NCI NIH HHS/United States
- 1RC1NS068204-01/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous