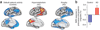Amyloid-beta-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks - PubMed (original) (raw)
Review
Amyloid-beta-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks
Jorge J Palop et al. Nat Neurosci. 2010 Jul.
Abstract
Alzheimer's disease is the most frequent neurodegenerative disorder and the most common cause of dementia in the elderly. Diverse lines of evidence suggest that amyloid-beta (Abeta) peptides have a causal role in its pathogenesis, but the underlying mechanisms remain uncertain. Here we discuss recent evidence that Abeta may be part of a mechanism controlling synaptic activity, acting as a positive regulator presynaptically and a negative regulator postsynaptically. The pathological accumulation of oligomeric Abeta assemblies depresses excitatory transmission at the synaptic level, but also triggers aberrant patterns of neuronal circuit activity and epileptiform discharges at the network level. Abeta-induced dysfunction of inhibitory interneurons likely increases synchrony among excitatory principal cells and contributes to the destabilization of neuronal networks. Strategies that block these Abeta effects may prevent cognitive decline in Alzheimer's disease. Potential obstacles and next steps toward this goal are discussed.
Figures
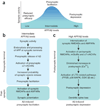
Figure 1. Presynaptic and postsynaptic regulation of synaptic transmission by Aβ
(a) Hypothetical relationship between Aβ level and synaptic activity. Intermediate levels of Aβ enhance synaptic activity presynaptically, whereas abnormally high or low levels of Aβ impair synaptic activity by inducing postsynaptic depression or reducing presynaptic efficacy, respectively. (b) Within a physiological range, small increases in Aβ primarily facilitate presynaptic functions, resulting in synaptic potentiation,. (c) At abnormally high levels, Aβ enhances LTD-related mechanisms, resulting in postsynaptic depression and loss of dendritic spines,,,.
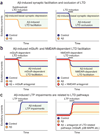
Figure 2. Depression of excitatory synapses by high Aβ levels requires activation of mGluR- and NMDAR-dependent LTD pathways
Panels depict summary diagrams; for details and actual data, see the papers cited below. (a) Aβ suppresses basal excitatory synaptic transmission (left and right), facilitates LTD after subthreshold LTD inductions (left) and occludes LTD (right), suggesting that Aβ-induced synaptic depression recruits LTD-like mechanisms. (b) Aβ facilitates LTD by inducing activation of mGluRs (left) and NMDARs (right). Aβ-induced facilitation of mGluR-dependent LTD is suppressed by mGluR antagonists (left, red), and Aβ-induced facilitation of NMDAR-dependent LTD is suppressed by NMDAR antagonists (right, red). (c) Aβ-induced LTP deficits depend on activation of LTD pathways. Aβ potently inhibits LTP (left). Blocking LTD-related signaling cascades with mGluR5 antagonists or an inhibitor of p38 MAPK (right, red) prevents Aβ-induced LTP impairments.
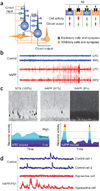
Figure 3. Pathologically elevated Aβ elicits abnormal patterns of neuronal activity in circuits and in wider networks in Alzheimer’s disease–related mouse models
(a) Neuronal circuits are formed by synaptic interactions between excitatory and inhibitory cells. Aβ might differentially affect excitatory (+) and inhibitory (−) synapses and cells, producing complex imbalances in circuit and network activity. (b) At the network level, high levels of Aβ increase network synchrony and elicit epileptiform activity, as illustrated here in EEG recordings from the left and right parietal cortex (LPC and RPC, respectively) of nontransgenic (NTG) controls (blue) and hAPP transgenic mice from line J20 (red). (c) hAPP mice show fluctuations in the neuronal expression of synaptic activity–dependent genes, suggesting network instability. Top: compared with NTG controls (left), hAPP-J20 mice show abnormally low (middle) or high (right) Arc expression in granule cells of the dentate gyrus. (Adapted with permission from refs. 8, 76). Percentages indicate the proportion of mice showing the different patterns of Arc expression. Such marked increases in Arc expression are typically caused by seizure activity. Bottom: interpretive diagram. Marked fluctuations in neuronal activity may directly impair cognition by reducing the time the network spends in activity patterns that promote normal cognitive functions. (d) In cortical circuits of mice monitored in vivo by calcium imaging, most neurons in NTG controls (blue traces) have an intermediate level of activity, whereas many neurons in hAPP/PS1 transgenic mice with high Aβ levels (red traces) are either hypoactive (top) or hyperactive (bottom). (Adapted with permission from ref. 9).
Figure 4. Radiological evidence for aberrant activity in neuronal networks of humans with Alzheimer’s disease
(a) The ‘default network’ (left) represents a group of brain regions that are activated at rest and deactivated during memory tasks in healthy controls. It includes the temporoparietal cortex, precuneus and posterior cingulate cortex. Individuals with Alzheimer’s disease (AD) show hypometabolism (middle) and atrophy (right) in these regions, possibly related to abnormal neuronal and synaptic activity. (Adapted with permission from ref. 64). (b) During memory encoding, individuals with Alzheimer’s disease show aberrant increases in default network activity compared with that in undemented controls. (Adapted with permission from ref. 11).
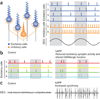
Figure 5. Aβ-induced dysfunction of inhibitory interneurons could promote aberrant synchrony in neural networks
(a) GABAergic interneurons regulate the activity of multiple excitatory principal cells (left). Action potentials (vertical strokes) of GABAergic interneurons and excitatory principal cells generate oscillatory electrical activity that can be detected by EEG recordings (right). Aβ-induced impairments of interneurons could disrupt this regulation and elicit abnormal patterns of network activity. (b) Hypothetical diagram depicting the firing pattern of cortical pyramidal neurons (cells 1–4) in nontransgenic (left) and hAPP transgenic (right) mice. Low excitatory neuronal activity or dysfunction of inhibitory interneurons can shift the activity of excitatory neuronal populations from a normal pattern (left) to a more synchronous pattern (right). Notably, increased synchrony resulting from enhanced GABAergic activity can also lead to epileptic activity. (c) Actual EEG recordings from nontransgenic (left) and hAPP transgenic (right) mice. There is increased synchrony, reflected by spikes and sharp waves, in the hAPP transgenic mouse.
Similar articles
- Oligomeric Aβ-induced synaptic dysfunction in Alzheimer's disease.
Tu S, Okamoto S, Lipton SA, Xu H. Tu S, et al. Mol Neurodegener. 2014 Nov 14;9:48. doi: 10.1186/1750-1326-9-48. Mol Neurodegener. 2014. PMID: 25394486 Free PMC article. Review. - Is Alzheimer's disease a result of presynaptic failure? Synaptic dysfunctions induced by oligomeric beta-amyloid.
Nimmrich V, Ebert U. Nimmrich V, et al. Rev Neurosci. 2009;20(1):1-12. doi: 10.1515/revneuro.2009.20.1.1. Rev Neurosci. 2009. PMID: 19526730 Review. - Alzheimer's disease-associated neurotoxic mechanisms and neuroprotective strategies.
Pereira C, Agostinho P, Moreira PI, Cardoso SM, Oliveira CR. Pereira C, et al. Curr Drug Targets CNS Neurol Disord. 2005 Aug;4(4):383-403. doi: 10.2174/1568007054546117. Curr Drug Targets CNS Neurol Disord. 2005. PMID: 16101556 Review. - Amyloid β: linking synaptic plasticity failure to memory disruption in Alzheimer's disease.
Ma T, Klann E. Ma T, et al. J Neurochem. 2012 Jan;120 Suppl 1(Suppl 1):140-148. doi: 10.1111/j.1471-4159.2011.07506.x. Epub 2011 Nov 28. J Neurochem. 2012. PMID: 22122128 Free PMC article. Review. - Cycles of aberrant synaptic sprouting and neurodegeneration in Alzheimer's and dementia with Lewy bodies.
Hashimoto M, Masliah E. Hashimoto M, et al. Neurochem Res. 2003 Nov;28(11):1743-56. doi: 10.1023/a:1026073324672. Neurochem Res. 2003. PMID: 14584828 Review.
Cited by
- Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-β.
Suberbielle E, Sanchez PE, Kravitz AV, Wang X, Ho K, Eilertson K, Devidze N, Kreitzer AC, Mucke L. Suberbielle E, et al. Nat Neurosci. 2013 May;16(5):613-21. doi: 10.1038/nn.3356. Epub 2013 Mar 24. Nat Neurosci. 2013. PMID: 23525040 Free PMC article. - False recognition in a mouse model of Alzheimer's disease: rescue with sensory restriction and memantine.
Romberg C, McTighe SM, Heath CJ, Whitcomb DJ, Cho K, Bussey TJ, Saksida LM. Romberg C, et al. Brain. 2012 Jul;135(Pt 7):2103-14. doi: 10.1093/brain/aws074. Epub 2012 Mar 30. Brain. 2012. PMID: 22466291 Free PMC article. - Discovery of a Novel Acetylcholinesterase Inhibitor by Fragment-Based Design and Virtual Screening.
Stavrakov G, Philipova I, Lukarski A, Atanasova M, Georgiev B, Atanasova T, Konstantinov S, Doytchinova I. Stavrakov G, et al. Molecules. 2021 Apr 3;26(7):2058. doi: 10.3390/molecules26072058. Molecules. 2021. PMID: 33916760 Free PMC article. - Nitric oxide signaling is recruited as a compensatory mechanism for sustaining synaptic plasticity in Alzheimer's disease mice.
Chakroborty S, Kim J, Schneider C, West AR, Stutzmann GE. Chakroborty S, et al. J Neurosci. 2015 Apr 29;35(17):6893-902. doi: 10.1523/JNEUROSCI.4002-14.2015. J Neurosci. 2015. PMID: 25926464 Free PMC article. - Emerging Roles of Inhibitor of Differentiation-1 in Alzheimer's Disease: Cell Cycle Reentry and Beyond.
Chen SD, Yang JL, Lin YC, Chao AC, Yang DI. Chen SD, et al. Cells. 2020 Jul 21;9(7):1746. doi: 10.3390/cells9071746. Cells. 2020. PMID: 32708313 Free PMC article. Review.
References
- Chapman PF, et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat. Neurosci. 1999;2:271–276. - PubMed
- Walsh DM, et al. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. - PubMed
- Kamenetz F, et al. APP processing and synaptic function. Neuron. 2003;37:925–937. - PubMed
- Cirrito JR, et al. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron. 2005;48:913–922. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS041787-11/NS/NINDS NIH HHS/United States
- NS041787/NS/NINDS NIH HHS/United States
- R01 NS041787/NS/NINDS NIH HHS/United States
- AG022074/AG/NIA NIH HHS/United States
- P01 AG022074/AG/NIA NIH HHS/United States
- P01 AG022074-09/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical