Ablation of mouse adult neurogenesis alters olfactory bulb structure and olfactory fear conditioning - PubMed (original) (raw)
Ablation of mouse adult neurogenesis alters olfactory bulb structure and olfactory fear conditioning
Matthew T Valley et al. Front Neurosci. 2009.
Abstract
Adult neurogenesis replenishes olfactory bulb (OB) interneurons throughout the life of most mammals, yet during this constant flux it remains unclear how the OB maintains a constant structure and function. In the mouse OB, we investigated the dynamics of turnover and its impact on olfactory function by ablating adult neurogenesis with an x-ray lesion to the sub-ventricular zone (SVZ). Regardless of the magnitude of the lesion to the SVZ, we found no change in the survival of young adult born granule cells (GCs) born after the lesion, and a gradual decrease in the population of GCs born before the lesion. After a lesion producing a 96% reduction of incoming adult born GCs to the OB, we found a diminished behavioral fear response to conditioned odor cues but not to audio cues. Interestingly, despite this behavioral deficit and gradual anatomical changes, we found no electrophysiological changes in the GC population assayed in vivo through dendro-dendritic synaptic plasticity and odor-evoked local field potential oscillations. These data indicate that turnover in the granule cell layer is generally decoupled from the rate of adult neurogenesis, and that OB adult neurogenesis plays a role in a wide behavioral system extending beyond the OB.
Keywords: amygdala; dendro-dendritic; freezing; granule cell; irradiation; plasticity; sub-ventricular zone; survival.
Figures
Figure 1
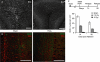
X-ray irradiation of the sub-ventricular zone produces a long-lasting reduction in cell proliferation. (A) Schematic showing the placement of the x-ray window (5 mm A-P × 13mm M-L). (B) BrdU positive nuclei along the SVZ were mostly eliminated weeks to months following irradiation. Scale bar = 100 μm. (C) X-ray targeting did not alter proliferation in the dentate gyrus observed using doublecortin, a marker for immature migrating neuroblasts. (D) A dose-dependent reduction in SVZ proliferation was produced by controlling the exposure time during the 3-day treatment. Significantly decreased proliferation relative to controls occurred at 10 and 15 Grays, and the maximally effective exposure was calculated to be 15 Grays (n = 3 per irradiated group, n = 9 for controls). (E) A significant depression in SVZ proliferation was observed up to 4 months in irradiated mice compared to age-matched controls (n = 3–6 for irradiated groups, n = 10 for pooled controls). **indicates p < 0.001 compared to non-irradiated control (grey) unpaired _t_-test, error bars indicate SEM.
Figure 2
Reduction in olfactory bulb neurogenesis is not accompanied by compensatory increase in new neuron survival. (A) The magnitude of olfactory bulb neurogenesis is qualitatively reflected by the amount of dcx(+) (Dcx) cells in the granule cell layer. (B) Quantification of cell survival was done by counting BrdU(+) nuclei, shown 4 weeks after irradiation. Scale bars: top 200 μm, bottom 50 μm. (C) Animals receiving SVZ irradiation (black) compared to non-irradiated controls (white) have an approximately 96% reduction in BrdU+ positive nuclei in the olfactory bulb measured 2 and 4 weeks after BrdU injection. Animals receiving a half dose of x-rays (grey) have an approximately 59% decrease in BrdU+ nuclei compared to non-irradiated controls (white, n = 3–4). Between 2 and 4 weeks after cell birth, the number of neurons decreased by approximately 39%. This decrease varied little according to the dose of irradiation (39.2% at 0 Gy; 44.4% at 7.5 Gy; 33.6% at 15 Gy). Error bars indicate SEM.
Figure 3
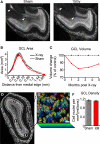
The number of cells in the granule cell layer is reduced following irradiation. (A) Sagittal sections of sham (left) and irradiated tissue (right). Sectioning the entire medial-lateral extent of the olfactory bulb followed by DAPI staining allowed surface area measurement of serial planes along the volume of interest. The rostral migratory stream is indicated by arrows, and is present in sham-treated tissue (left) but nearly absent in irradiated tissue (right). (B) Cross-sectional area of the granule cell layer for data grouped across all time points. (C) The volume encompassed by the edge of the GCL declined in the x-rayed condition (solid red line) compared to controls (dotted line) between 1 and 5 months after irradiation (values represent group means, for statistics see Table 1). (D) Example density counting windows placed in an irradiated GCL. (E) Implementation of the optical disector to estimate cell density from high-magnification _z_-stacks of GCL nuclei false-colored according to _z_-depth, scale = 10 um. (F) Density of granule cell layer from mice receiving sham treatment (grey), and irradiation (red). This data represents the average of multiple anatomical regions, and durations between 1 and 5 months after irradiation. We observed no trend in density over time. All error bars indicate SEM.
Figure 4
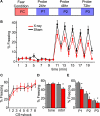
Irradiated mice show normal acquisition of fear conditioning, but display less odor-cued freezing. (A) An illustration of the timeline of testing: Fear Conditioning consists of a 40-min session that includes 8 CS + shock pairings with a variable inter-trial interval of 4–6 min. At 24, 48, and 72 h after conditioning a 20-min probe session is conducted in a novel context. (B) Time course of a probe of odor-cued conditioned freezing response. Plotted is the mean amount of freezing as a percentage of each minute in the 20-min probe; all three probe sessions are included in the calculation of mean percent freezing. All mice conditioned to odor are grouped: XR n = 17 (red) and sham n = 18 (black). The increase in freezing in the minute immediately following CS (minutes 11, 13, 15, 17, and 19) is the “post-CS minute” that is used in subsequent analysis of freezing. (C) Acquisition of freezing response during conditioning. The average percent freezing is plotted for XR (n = 17) and sham (n = 18) mice; here the percent freezing is during the 2 min immediately following each CS + shock pairing. Only mice conditioned to odor are represented; mice conditioned to an audio tone displayed a similar pattern for acquisition of freezing behavior (data not shown). (D) Members of a cohort of mice tested 26-weeks post-irradiation were conditioned to a 2 kHz audio tone rather than odor. In this graph mean percent freezing from all three probe sessions for the +26-week cohort is plotted; the reduced freezing displayed by the XR group (factorial ANOVA n = 10, p < 0.05) in response to a conditioned odor was not observed for mice conditioned to an audio tone (n = 10). (E) The average percent freezing for the “post-CS minute” during each of the three probe sessions is plotted. XR mice (n = 17) freeze less than Sham mice (n = 18); repeated measures ANOVA p < 0.05. In factorial ANOVA P1 and P2 are p < 0.05, and for P3 p = 0.0627. All error bars represent SEM.
Figure 5
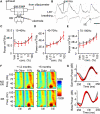
Loss of neurogenesis does not change the activation and plasticity of the dendro-dendritic synapse in-vivo. (A) Schematic of the stimulation paradigm, with a diagram of the dendro-dendritic circuit between mitral cells (MC) and granule cells (GC). (B) The waveform following stimulation has three distinct phases corresponding to the mitral cell compound action potential (cAP), the field EPSP (fEPSP) originating in the granule cells, and the reciprocal field IPSP (fIPSP) in mitral cells. (C) Input-output function relating intensity of 100 μs LOT stimulation to fEPSP magnitude from all animals. The current that evokes a half-maximal response is depicted on the right. (D–F) Paired-pulse stimulation of the synapse at maximal intensity, and over a range of inter-stimulus intervals (ISI) from 10 ms to 1 s. This paradigm reveals minor facilitation (values > 1) in the granule cell fEPSP (left) and large depression (values < 1) in the mitral cell fIPSP (right).
Figure 6
Oscillatory dynamics in the olfactory bulb are unchanged months following a reduction of neurogenesis. (A) In vivo recording paradigm, identical to that in Figure 4, highlighting the odor delivery apparatus. (B) Local field potential (LFP) traces (top) are recorded alongside the breathing rate (bottom). Gamma frequency (40–100 Hz) oscillations are highlighted emerging after a breath. The transition between inhalation and exhalation (I/E) is used as the trigger for all analyses and odor delivery (Scale = 250 ms). Spectral density of beta (C), gamma (D) and high gamma (E) LFP oscillations measured after stimulation with increasing concentrations of the odor Amyl Acetate with vehicle DMSO. Recordings were performed 1–2 months after sham (black) or irradiation (red) treatment (n = 9–10 per group). (F) Frequency-time spectrogram showing fine temporal analysis of oscillatory power over 500 ms centered around a breath. Each spectrogram represents the average of its dataset (n = 9–10 for all groups). (G) Extracting beta (10–40 Hz) and gamma (40–70 Hz) band-power from the 1–2 month spectrograms shows how stimulation with 10% Amyl Acetate produces a distinct profile of power within the breathing cycle, but without significant differences between groups. The average ±SEM is depicted for sham (grey) and irradiated (red) animals.
Similar articles
- CPEB4-Dependent Neonate-Born Granule Cells Are Required for Olfactory Discrimination.
Tseng CS, Chou SJ, Huang YS. Tseng CS, et al. Front Behav Neurosci. 2019 Jan 23;13:5. doi: 10.3389/fnbeh.2019.00005. eCollection 2019. Front Behav Neurosci. 2019. PMID: 30728769 Free PMC article. - The Functional Role of Olfactory Bulb Granule Cell Subtypes Derived From Embryonic and Postnatal Neurogenesis.
Takahashi H, Yoshihara S, Tsuboi A. Takahashi H, et al. Front Mol Neurosci. 2018 Jul 5;11:229. doi: 10.3389/fnmol.2018.00229. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30034321 Free PMC article. Review. - A Subtype of Olfactory Bulb Interneurons Is Required for Odor Detection and Discrimination Behaviors.
Takahashi H, Ogawa Y, Yoshihara S, Asahina R, Kinoshita M, Kitano T, Kitsuki M, Tatsumi K, Okuda M, Tatsumi K, Wanaka A, Hirai H, Stern PL, Tsuboi A. Takahashi H, et al. J Neurosci. 2016 Aug 3;36(31):8210-27. doi: 10.1523/JNEUROSCI.2783-15.2016. J Neurosci. 2016. PMID: 27488640 Free PMC article. - VEGF is required for dendritogenesis of newly born olfactory bulb interneurons.
Licht T, Eavri R, Goshen I, Shlomai Y, Mizrahi A, Keshet E. Licht T, et al. Development. 2010 Jan;137(2):261-71. doi: 10.1242/dev.039636. Development. 2010. PMID: 20040492 - Regulation of adult neurogenesis by GABAergic transmission: signaling beyond GABAA-receptors.
Pallotto M, Deprez F. Pallotto M, et al. Front Cell Neurosci. 2014 Jun 20;8:166. doi: 10.3389/fncel.2014.00166. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24999317 Free PMC article. Review.
Cited by
- Adult Olfactory Bulb Neurogenesis.
Lledo PM, Valley M. Lledo PM, et al. Cold Spring Harb Perspect Biol. 2016 Aug 1;8(8):a018945. doi: 10.1101/cshperspect.a018945. Cold Spring Harb Perspect Biol. 2016. PMID: 27235474 Free PMC article. Review. - Female mice lacking cholecystokinin 1 receptors have compromised neurogenesis, and fewer dopaminergic cells in the olfactory bulb.
Sui Y, Vermeulen R, Hökfelt T, Horne MK, Stanić D. Sui Y, et al. Front Cell Neurosci. 2013 Mar 1;7:13. doi: 10.3389/fncel.2013.00013. eCollection 2013. Front Cell Neurosci. 2013. PMID: 23459364 Free PMC article. - Epigenetic control of neurotransmitter expression in olfactory bulb interneurons.
Banerjee K, Akiba Y, Baker H, Cave JW. Banerjee K, et al. Int J Dev Neurosci. 2013 Oct;31(6):415-23. doi: 10.1016/j.ijdevneu.2012.11.009. Epub 2012 Dec 3. Int J Dev Neurosci. 2013. PMID: 23220178 Free PMC article. Review. - Early activation of microglia triggers long-lasting impairment of adult neurogenesis in the olfactory bulb.
Lazarini F, Gabellec MM, Torquet N, Lledo PM. Lazarini F, et al. J Neurosci. 2012 Mar 14;32(11):3652-64. doi: 10.1523/JNEUROSCI.6394-11.2012. J Neurosci. 2012. PMID: 22423088 Free PMC article. - Trehalose ameliorates prodromal non-motor deficits and aberrant protein accumulation in a rotenone-induced mouse model of Parkinson's disease.
Moon SH, Kwon Y, Huh YE, Choi HJ. Moon SH, et al. Arch Pharm Res. 2022 Jun;45(6):417-432. doi: 10.1007/s12272-022-01386-2. Epub 2022 May 26. Arch Pharm Res. 2022. PMID: 35618982
References
LinkOut - more resources
Full Text Sources
Miscellaneous