Radiosensitization and stromal imaging response correlates for the HIF-1 inhibitor PX-478 given with or without chemotherapy in pancreatic cancer - PubMed (original) (raw)
Radiosensitization and stromal imaging response correlates for the HIF-1 inhibitor PX-478 given with or without chemotherapy in pancreatic cancer
David L Schwartz et al. Mol Cancer Ther. 2010 Jul.
Abstract
Growing tumors are hypoxic and respond to microenvironmental stress through increased expression of the hypoxia inducible factor-1alpha (HIF-1alpha) transcription factor, resulting in an adaptive switch to glycolytic metabolism, angiogenic signaling, survival, and metastasis. HIF-1alpha expression is associated with tumor resistance to cytotoxic therapy and inferior patient outcomes. Pancreatic cancer is the most hypoxic of all solid tumors and remains refractory to current chemoradiotherapy. We have seen nuclear HIF-1alpha in 88% of human pancreatic ductal carcinoma but in only 16% of normal pancreas. Stroma adjacent to the pancreatic ductal carcinoma also showed HIF-1alpha in 43% of cases. We investigated the novel selective HIF-1alpha inhibitor PX-478 on in vitro and in vivo radiation response of human pancreatic cancer models. Inhibition of HIF-1alpha by PX-478 increased cell killing by radiation. In mice with Panc-1, CF-PAC-1, or SU.86.86 pancreatic xenografts, concurrent administration of PX-478 potentiated the antitumor effects of fractionated radiation, with or without combined treatment with 5-fluorouracil or gemcitabine. Alternative sequencing of PX-478 with fractionated radiotherapy suggests optimal radiosensitization with concurrent or neoadjuvant administration of drug. Early tumor responses to combined PX-478/radiation treatment could be rapidly and repeatedly quantified by vascular imaging biomarkers. Dual-tracer dynamic contrast enhanced-magnetic resonance imaging and ultrasound imaging discriminated response to combined treatment prior to detection of differences in anatomic tumor size at 10 days posttreatment. Therefore, PX-478 is a mechanistically appealing and potentially clinically relevant enhancer of pancreatic cancer radiosensitivity, inhibiting tumor and stromal HIF-1 proangiogenic signaling and reducing the innate radiation resistance of hypoxic tumor cells.
(c)2010 AACR.
Figures
Figure 1
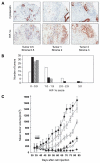
Immunohistochemistry of HIF-1 in patient tumors and PX-478 potentiation of the antitumor activity of fractionated radiation in Panc-1 tumor xenografts. A, typical staining of cytokeratin and HIF-1α. Left to right, two moderately well-differentiated ductal carcinomas and a moderately differentiated acinar carcinoma. The numbers are HIF-1α staining on a scale of 0 to 3 for the pancreatitis/tumor and for the adjacent stroma. B, histogram showing numbers of tumors in each staining range. Open bars, PDC (42 evaluable); filled bars, normal pancreas (42 evaluable); rough cross hatching, stroma adjacent to tumor (32 evaluable); fine cross hatching, stroma distant to tumor (30 evaluable). Stroma did not include blood vessel staining. C, female SCID mice with Panc-1 pancreatic tumor xenografts, 8 mice per group, were treated with vehicle alone (●);1 Gy radiation daily for 5 days (□); PX-478 10 mg/kg orally daily for 5 days (△); PX-478 20 mg/kg orally daily for 5 days (▲); PX-478 10 mg/kg orally daily for 5 days 1 hour before Gy radiation daily 1 for 5 days (◇); and PX-478 20 mg/kg orally daily for 5 days 1 hour before 1 Gy radiation daily for 5 days (◆). Arrows, daily dosing and/or radiation treatment. Values are the mean of 8 mice per group; bars, SE of mean.
Figure 2
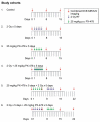
PX-478/radiation sequencing study treatment cohorts. Female SCID mice with Panc-1 pancreatic tumor xenografts, 8 mice per group, were treated with single-modality treatment or concurrent PX-478 25 mg/kg orally and 2 Gy radiation daily for 5 days per the indicated sequences. Timing of serial MRI and ultrasound measurements of each cohort is also indicated.
Figure 3
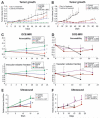
Tumor growth curves, DCE-MRI permeability/vascular volume fraction measurements, and power Doppler ultrasound measurements of tumor blood flow. Female SCID mice with Panc-1 pancreatic tumor xenografts, 8 mice per group, were treated with single-modality treatment or concurrent PX-478 25 mg/kg orally and 2 Gy radiation daily for 5 days (A, C, E), or over two weeks with sequenced PX-478 and radiation (XRT; B, D, F), as indicated. Statistical comparisons of results at each time point are with untreated controls, as reported as mean values ± SD; *, P < 0.05; **, P < 0.01.
Figure 4
Representative serial DCE-MRI permeability/vascular volume fraction maps for indicated PX-478/radiation sequencing study treatment cohorts. Female SCID mice with Panc-1 pancreatic tumor xenografts were treated with single-modality treatment or concurrent PX-478 25 mg/kg orally and 2 Gy radiation daily for 5 days (A) or over two weeks with sequenced PX-478 and radiation (B).
Similar articles
- The selective hypoxia inducible factor-1 inhibitor PX-478 provides in vivo radiosensitization through tumor stromal effects.
Schwartz DL, Powis G, Thitai-Kumar A, He Y, Bankson J, Williams R, Lemos R, Oh J, Volgin A, Soghomonyan S, Nishii R, Alauddin M, Mukhopadhay U, Peng Z, Bornmann W, Gelovani J. Schwartz DL, et al. Mol Cancer Ther. 2009 Apr;8(4):947-58. doi: 10.1158/1535-7163.MCT-08-0981. Mol Cancer Ther. 2009. PMID: 19372568 Free PMC article. - Inhibition of HIF-1α by PX-478 enhances the anti-tumor effect of gemcitabine by inducing immunogenic cell death in pancreatic ductal adenocarcinoma.
Zhao T, Ren H, Jia L, Chen J, Xin W, Yan F, Li J, Wang X, Gao S, Qian D, Huang C, Hao J. Zhao T, et al. Oncotarget. 2015 Feb 10;6(4):2250-62. doi: 10.18632/oncotarget.2948. Oncotarget. 2015. PMID: 25544770 Free PMC article. - PX-478, an inhibitor of hypoxia-inducible factor-1alpha, enhances radiosensitivity of prostate carcinoma cells.
Palayoor ST, Mitchell JB, Cerna D, Degraff W, John-Aryankalayil M, Coleman CN. Palayoor ST, et al. Int J Cancer. 2008 Nov 15;123(10):2430-7. doi: 10.1002/ijc.23807. Int J Cancer. 2008. PMID: 18729192 Free PMC article. - A novel approach to cancer therapy using PX-478 as a HIF-1α inhibitor.
Lee K, Kim HM. Lee K, et al. Arch Pharm Res. 2011 Oct;34(10):1583-5. doi: 10.1007/s12272-011-1021-3. Arch Pharm Res. 2011. PMID: 22076756 Review. - HIF-1alpha and cancer therapy.
Koh MY, Spivak-Kroizman TR, Powis G. Koh MY, et al. Recent Results Cancer Res. 2010;180:15-34. doi: 10.1007/978-3-540-78281-0_3. Recent Results Cancer Res. 2010. PMID: 20033376 Review.
Cited by
- On the Evaluation of a Novel Hypoxic 3D Pancreatic Cancer Model as a Tool for Radiotherapy Treatment Screening.
Wishart G, Gupta P, Nisbet A, Schettino G, Velliou E. Wishart G, et al. Cancers (Basel). 2021 Dec 2;13(23):6080. doi: 10.3390/cancers13236080. Cancers (Basel). 2021. PMID: 34885188 Free PMC article. - Activation of Hif1α by the prolylhydroxylase inhibitor dimethyoxalyglycine decreases radiosensitivity.
Ayrapetov MK, Xu C, Sun Y, Zhu K, Parmar K, D'Andrea AD, Price BD. Ayrapetov MK, et al. PLoS One. 2011;6(10):e26064. doi: 10.1371/journal.pone.0026064. Epub 2011 Oct 7. PLoS One. 2011. PMID: 22016813 Free PMC article. - Carbogen gas and radiotherapy outcomes in prostate cancer.
Yip K, Alonzi R. Yip K, et al. Ther Adv Urol. 2013 Feb;5(1):25-34. doi: 10.1177/1756287212452195. Ther Adv Urol. 2013. PMID: 23372608 Free PMC article. - Opportunities and challenges facing biomarker development for personalized head and neck cancer treatment.
Lucs A, Saltman B, Chung CH, Steinberg BM, Schwartz DL. Lucs A, et al. Head Neck. 2013 Feb;35(2):294-306. doi: 10.1002/hed.21975. Epub 2012 Jan 27. Head Neck. 2013. PMID: 22287320 Free PMC article. Review. - Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions.
Chen Z, Han F, Du Y, Shi H, Zhou W. Chen Z, et al. Signal Transduct Target Ther. 2023 Feb 17;8(1):70. doi: 10.1038/s41392-023-01332-8. Signal Transduct Target Ther. 2023. PMID: 36797231 Free PMC article. Review.
References
- Brown JM. Exploiting the hypoxic cancer cell: mechanisms and therapeutic strategies. Mol Med Today. 2000;6:157–62. - PubMed
- Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities and (problems) for cancer therapy. Cancer Res. 1998;58:1408–16. - PubMed
- Kaelin WG. Proline hydroxylation and gene expression. Ann Rev Biochem. 2005;74:115–28. - PubMed
- Semenza GL. HIF-1 and tumor progession: pathophysiology and therapeutics. Trends Mol Med. 2002;8:S62–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016672/CA/NCI NIH HHS/United States
- K08 DE018061-04/DE/NIDCR NIH HHS/United States
- R01 CA098920-07/CA/NCI NIH HHS/United States
- K08 DE018061/DE/NIDCR NIH HHS/United States
- P30 CA016672-34/CA/NCI NIH HHS/United States
- P01 CA109552-050002/CA/NCI NIH HHS/United States
- U24 CA126577/CA/NCI NIH HHS/United States
- 016672/PHS HHS/United States
- U24 CA126577-05/CA/NCI NIH HHS/United States
- P50 CA095060/CA/NCI NIH HHS/United States
- P01 CA017094/CA/NCI NIH HHS/United States
- 017094/PHS HHS/United States
- P01 CA017094-290031/CA/NCI NIH HHS/United States
- P01 CA109552/CA/NCI NIH HHS/United States
- 095060/PHS HHS/United States
- R01 CA098920/CA/NCI NIH HHS/United States
- 126577/PHS HHS/United States
- CA109552/CA/NCI NIH HHS/United States
- P50 CA095060-080003/CA/NCI NIH HHS/United States
- 095920/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical