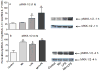Manganese modulation of MAPK pathways: effects on upstream mitogen activated protein kinase kinases and mitogen activated kinase phosphatase-1 in microglial cells - PubMed (original) (raw)
Manganese modulation of MAPK pathways: effects on upstream mitogen activated protein kinase kinases and mitogen activated kinase phosphatase-1 in microglial cells
Patrick L Crittenden et al. J Appl Toxicol. 2011 Jan.
Abstract
Multiple studies demonstrate that manganese (Mn) exposure potentiates inflammatory mediator output from activated glia; this increased output is associated with enhanced mitogen activated protein kinase (MAPK: p38, ERK and JNK) activity. We hypothesized that Mn activates MAPK by activating the kinases upstream of MAPK, i.e. MKK-3/6, MKK-1/2 and MKK-4 (responsible for activation of p38, ERK, and JNK, respectively), and/or by inhibiting a major phosphatase responsible for MAPK inactivation, MKP-1. Exposure of N9 microglia to Mn (250 µm), LPS (100 ng ml⁻¹) or Mn + LPS increased MKK-3/6 and MKK-4 activity at 1 h; the effect of Mn + LPS on MKK-4 activation was greater than the rest. At 4 h, Mn, LPS, and Mn + LPS increased MKK-3/6 and MKK-1/2 phosphorylation, whereas MKK-4 was activated only by Mn and Mn + LPS. Besides activating MKK-4 via Ser257/Thr261 phosphorylation, Mn (4 h) prevented MKK-4's phosphorylation on Ser80, which negatively regulates MKK-4 activity. Exposure to Mn or Mn + LPS (1 h) decreased both mRNA and protein expression of MKP-1, the negative MAPK regulator. In addition, we observed that at 4 h, but not at 1 h, a time point coinciding with increased MAPK activity, Mn + LPS markedly increased TNF-α, IL-6 and Cox-2 mRNA, suggesting a delayed effect. The fact that all three major groups of MKKs, MKK-1/2, MKK-3/6 and MKK-4, are activated by Mn suggests that Mn-induced activation of MAPK occurs via traditional mechanisms, which perhaps involve the MAPKs furthest upstream, MKKKs (MAP3Ks). In addition, for all MKKs, Mn-induced activation was persistent at least for 4 h, indicating a long-term effect.
Copyright © 2010 John Wiley & Sons, Ltd.
Figures
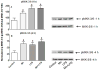
Figure 1
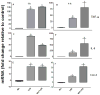
Effects of Mn and/or LPS on MKK-3/6 activity. Shown are quantification and representative western blots of phosphorylated MKK-3/6 (pMKK-3/6; Ser 189) and total MKK-3/6 in N9 microglia following exposure to vehicle, 250 μM Mn, 100 ng/ml LPS, or 250 μM Mn + 100 ng/ml LPS for 1 (A) and 4 (B) h. Densitometric data were normalized as a ratio of phosphorylated to total MKK-3/6 protein. All data points represent means ± SEM from at least 3 independent experiments. Data were analyzed with ANOVA and means were separated using Fisher’s LSD multiple comparison post hoc test. a Letters denote statistically significant difference from control at p ≤ 0.05.
Figure 2
Effects of Mn and/or LPS on MKK-1/2 activity. Shown are quantification and representative western blots of phosphorylated MKK-1/2 (pMKK-1/2; Ser 218/Ser 222) and total MKK-1/2 in N9 microglia following exposure to vehicle, 250 μM Mn, 100 ng/ml LPS, or 250 μM Mn + 100 ng/ml LPS for 1 (A) and 4 (B) h. Densitometric data were normalized as a ratio of phosphorylated to total MKK-1/2 protein. All data points represent means ± SEM from at least 3 independent experiments. Data were analyzed with ANOVA and means were separated using Fisher’s LSD multiple comparison post hoc test. a Letters denote statistically significant difference from control at p ≤ 0.05.
Figure 3
Effects of Mn and/or LPS on MKK-4 activity modulated at Ser 257/Thr 261. Shown are quantification and representative western blots of Ser 257/Thr 261-phosphorylated MKK-4 (pMKK-4; Ser 257/Thr 261) and total MKK-4 in N9 microglia following exposure to vehicle, 250 μM Mn, 100 ng/ml LPS, or 250 μM Mn + 100 ng/ml LPS for 1 (A) and 4 (B) h. Densitometric data were normalized as a ratio of Ser 257/Thr 261-pMKK-4 to total MKK-4 protein. All data points represent means ± SEM from at least 3 independent experiments. Data were analyzed with ANOVA and means were separated using Fisher’s LSD multiple comparison post hoc test. a ,b Letters denote statistically significant difference from control, with different letters also being different from each other at p ≤ 0.05.
Figure 4
Effects of Mn and/or LPS on MKK-4 activity modulated at Ser 80. Shown are quantification and representative western blots of Ser 80-phosphorylated MKK-4 (pMKK-4; Ser 80) and total MKK-4 in N9 microglia following exposure to vehicle, 250 μM Mn, 100 ng/ml LPS, or 250 μM Mn + 100 ng/ml LPS for 1 (A) and 4 (B) h. Densitometric data were normalized as a ratio of Ser 80-pMKK-4 to total MKK-4 protein. All data points represent means ± SEM from at least 3 independent experiments. Data were analyzed with ANOVA and means were separated using Fisher’s LSD multiple comparison post hoc test. a Letters denote statistically significant difference from control at p ≤ 0.05.
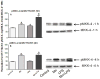
Figure 5
Effects of Mn and/or LPS on MKP-1. Shown are representative western blots and quantification of MKP-1 protein (A) and mRNA (B) levels following exposure to vehicle, 250 μM Mn, 100 ng/ml LPS, or 250 μM Mn + 100 ng/ml LPS for 1 h. Densitometric data were normalized as a ratio of MPK-1 to α-tubulin protein. All data points represent means ± SEM from at least 3 (protein) or 4 (mRNA) independent experiments. Data were analyzed with ANOVA and means were separated using Fisher’s LSD multiple comparison post hoc test. a ,b,c Letters denote statistically significant difference from control, with different letters also being different from each other at p ≤ 0.05.
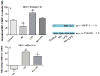
Figure 6
Fold change (up/down from vehicle control) in TNF-α (A and B), IL-6 (C and D), and Cox-2 (E and F) mRNA expression following exposure to 250 μM Mn, 100 ng/ml LPS, or 250μM Mn + 100 ng/ml LPS for 1 (left-hand graphs) and 4 (right-hand graphs) h. All data points represent means ± SEM from 4 independent experiments. Data were analyzed with ANOVA and means were separated using Fisher’s LSD multiple comparison post hoc test. a ,b Letters denote statistically significant difference from control, with different letters also being different from each other at p ≤ 0.05.
Figure 7
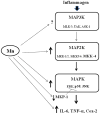
Simplified MAPK signaling pathway diagram depicting places where Mn has (demonstrated previously and in this study) an effect. In microglial cells, this pathway can be activated by a variety of stimuli, including inflammagens, such as LPS. Arrows beside components of the pathway denote increases or decreases caused by Mn (↑, ↓, respectively). Potential effects of Mn on MAP3K have not been studied yet and are hence marked with a question mark (?). Abbreviations: Mitogen activated protein kinase kinase kinase (MAP3K, i.e., MLK3, TAK, ASK1); mitogen activated protein kinase kinase (MAP2K, i.e. MKK-1/2, MKK-4, MKK-3/6); mitogen activated protein kinase (MAPK, i.e., extracellular regulated protein kinase [ERK], p38, c-Jun N-terminal kinase [JNK]); mitogen activated kinase phosphatase-1 (MKP-1); interleukin-6 (IL-6); tumor necrosis factor-alpha (TNF-α ); cyclooxygenase-2 (Cox-2).
Similar articles
- Manganese-induced potentiation of in vitro proinflammatory cytokine production by activated microglial cells is associated with persistent activation of p38 MAPK.
Crittenden PL, Filipov NM. Crittenden PL, et al. Toxicol In Vitro. 2008 Feb;22(1):18-27. doi: 10.1016/j.tiv.2007.07.004. Epub 2007 Jul 21. Toxicol In Vitro. 2008. PMID: 17845838 Free PMC article. - Mitogen-activated protein kinases and nuclear factor-kappaB regulate Helicobacter pylori-mediated interleukin-8 release from macrophages.
Bhattacharyya A, Pathak S, Datta S, Chattopadhyay S, Basu J, Kundu M. Bhattacharyya A, et al. Biochem J. 2002 Nov 15;368(Pt 1):121-9. doi: 10.1042/BJ20020555. Biochem J. 2002. PMID: 12150710 Free PMC article. - Regulation of JNK and p38 MAPK in the immune system: signal integration, propagation and termination.
Huang G, Shi LZ, Chi H. Huang G, et al. Cytokine. 2009 Dec;48(3):161-9. doi: 10.1016/j.cyto.2009.08.002. Epub 2009 Sep 8. Cytokine. 2009. PMID: 19740675 Free PMC article. Review. - Regulation of Mitogen-Activated Protein Kinase Signaling Pathways by the Ubiquitin-Proteasome System and Its Pharmacological Potential.
Mathien S, Tesnière C, Meloche S. Mathien S, et al. Pharmacol Rev. 2021 Oct;73(4):263-296. doi: 10.1124/pharmrev.120.000170. Pharmacol Rev. 2021. PMID: 34732541 Review.
Cited by
- Manganese Is Essential for Neuronal Health.
Horning KJ, Caito SW, Tipps KG, Bowman AB, Aschner M. Horning KJ, et al. Annu Rev Nutr. 2015;35:71-108. doi: 10.1146/annurev-nutr-071714-034419. Epub 2015 May 13. Annu Rev Nutr. 2015. PMID: 25974698 Free PMC article. Review. - Manganese Acts upon Insulin/IGF Receptors to Phosphorylate AKT and Increase Glucose Uptake in Huntington's Disease Cells.
Bryan MR, Nordham KD, Rose DIR, O'Brien MT, Joshi P, Foshage AM, Gonçalves FM, Nitin R, Uhouse MA, Aschner M, Bowman AB. Bryan MR, et al. Mol Neurobiol. 2020 Mar;57(3):1570-1593. doi: 10.1007/s12035-019-01824-1. Epub 2019 Dec 4. Mol Neurobiol. 2020. PMID: 31797328 Free PMC article. - In vivo manganese exposure modulates Erk, Akt and Darpp-32 in the striatum of developing rats, and impairs their motor function.
Cordova FM, Aguiar AS Jr, Peres TV, Lopes MW, Gonçalves FM, Remor AP, Lopes SC, Pilati C, Latini AS, Prediger RD, Erikson KM, Aschner M, Leal RB. Cordova FM, et al. PLoS One. 2012;7(3):e33057. doi: 10.1371/journal.pone.0033057. Epub 2012 Mar 13. PLoS One. 2012. PMID: 22427945 Free PMC article. - Manganese potentiates LPS-induced heme-oxygenase 1 in microglia but not dopaminergic cells: role in controlling microglial hydrogen peroxide and inflammatory cytokine output.
Dodd CA, Filipov NM. Dodd CA, et al. Neurotoxicology. 2011 Dec;32(6):683-92. doi: 10.1016/j.neuro.2011.09.002. Epub 2011 Sep 25. Neurotoxicology. 2011. PMID: 21963524 Free PMC article. - In vitro manganese exposure disrupts MAPK signaling pathways in striatal and hippocampal slices from immature rats.
Peres TV, Pedro DZ, de Cordova FM, Lopes MW, Gonçalves FM, Mendes-de-Aguiar CB, Walz R, Farina M, Aschner M, Leal RB. Peres TV, et al. Biomed Res Int. 2013;2013:769295. doi: 10.1155/2013/769295. Epub 2013 Nov 13. Biomed Res Int. 2013. PMID: 24324973 Free PMC article.
References
- Bae JH, Jang BC, Suh SI, Ha E, Baik HH, Kim SS, Lee MY, Shin DH. Manganese induces inducible nitric oxide synthase (iNOS) expression via activation of both MAP kinase and PI3K/Akt pathways in BV2 microglial cells. Neurosci Lett. 2006;398(1–2):151–4. - PubMed
- Barhoumi R, Faske J, Liu X, Tjalkens RB. Manganese potentiates lipopolysaccharide-induced expression of NOS2 in C6 glioma cells through mitochondrial–dependent activation of nuclear factor kappaB. Brain Res Mol Brain Res. 2004;122(2):167–79. - PubMed
- Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci. 1998;18(5):1633–41. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- ES016965/ES/NIEHS NIH HHS/United States
- R01 ES016965-01A1/ES/NIEHS NIH HHS/United States
- K22 ES011654-03/ES/NIEHS NIH HHS/United States
- ES011654/ES/NIEHS NIH HHS/United States
- R01 ES016965/ES/NIEHS NIH HHS/United States
- K22 ES011654/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous