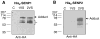Distribution and paralogue specificity of mammalian deSUMOylating enzymes - PubMed (original) (raw)
Distribution and paralogue specificity of mammalian deSUMOylating enzymes
Nagamalleswari Kolli et al. Biochem J. 2010.
Abstract
The covalent attachment of SUMO (small ubiquitin-like protein modifier) to target proteins results in modifications in their activity, binding interactions, localization or half-life. The reversal of this modification is catalysed by SENPs (SUMO-specific processing proteases). Mammals contain four SUMO paralogues and six SENP enzymes. In the present paper, we describe a systematic analysis of human SENPs, integrating estimates of relative selectivity for SUMO1 and SUMO2, and kinetic measurements of recombinant C-terminal cSENPs (SENP catalytic domains). We first characterized the reaction of each endogenous SENP and cSENPs with HA-SUMO-VS [HA (haemagglutinin)-tagged SUMO-vinyl sulfones], active-site-directed irreversible inhibitors of SENPs. We found that all cSENPs and endogenous SENP1 react with both SUMO paralogues, whereas all other endogenous SENPs in mammalian cells and tissues display high selectivity for SUMO2-VS. To obtain more quantitative data, the kinetic properties of purified cSENPs were determined using SUMO1- or SUMO2-AMC (7-amino-4-methylcoumarin) as substrate. All enzymes bind their respective substrates with high affinity. cSENP1 and cSENP2 process either SUMO substrate with similar affinity and catalytic efficiency; cSENP5 and cSENP6 show marked catalytic specificity for SUMO2 as measured by Km and kcat, whereas cSENP7 works only on SUMO2. Compared with cSENPs, recombinant full-length SENP1 and SENP2 show differences in SUMO selectivity, indicating that paralogue specificity is influenced by the presence of the variable N-terminal domain of each SENP. Our data suggest that SUMO2 metabolism is more dynamic than that of SUMO1 since most SENPs display a marked preference for SUMO2.
Figures
Figure 1. Human SENP proteases
The white bars represent nonconserved N-terminal regions. The conserved catalytic domain is shown in grey. Note the insertions in the catalytic domains of SENPs 6 and 7 shown in black. Green circle indicates SUMO-binding Motifs.
Figure 2. Irreversible labeling of the catalytic domains of SENPs with HA-SUMO-VS
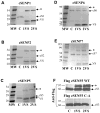
A–E) Recombinant cSENPs (cS) 1, 2, 5, 6, and 7 were reacted with HA-SUMO1-VS and HA-SUMO2-VS for 15 minutes at 25°C. Samples were run on 12% SDS-PAGE and visualized by Imperial protein stain. Asterisks show adduct formation with all cSENPs indicates reactivity with SUMO1-VS and SUMO2-VS. F) FLAG-cSENP3WT and FLAG-cSENP3C-A (active site mutation) were reacted with HA-SUMO1-VS and SUMO2-VS for 15 minutes at 25°C. The reactions were monitored by immunoblotting using anti-FLAG antibody. FLAG-cSENP3 WT formed adduct with both SUMO1-VS and SUMO2-VS, whereas FLAG-cSENP3CA did not form adducts. Asterisks indicate the vinylsulfone adducts.
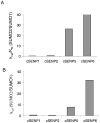
Figure 3. Kinetic constants for cSENP catalyzed hydrolysis of SUMO-AMC Summary of steady state kinetic constants for the hydrolysis of SUMO1-AMC and SUMO2-AMC by the catalytic domains of human SENPs
A) (kcat/KM) ratio, SUMO2/SUMO1. B) kcat ratio, SUMO2/SUMO1
Figure 4. Paralog preference of endogenous SENPs
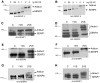
A–B) HEK293 lysate (30μg) was titrated with HA-SUMO2-VS and reacted for 15 minutes. Adduct formation was monitored by 12% SDS-PAGE and western blotting with anti-SENP3 and SENP5 antibodies in separate experiments. Lanes 5–6 of panel A indicates that all endogenous SENP3 in the lysate reacted with SUMO2-VS. Similarly in Figure 4B lanes 5–6 show that all endogenous SENP5 reacted with SUMO2-VS. C–H) HeLa cell lysate was incubated with HA-SUMO1-VS or HA-SUMO2-VS for 15 minutes. The incorporation of SUMO-VS into SUMO-specific proteases was monitored by 12% SDS-PAGE and immunoblotting with anti-SENPs 1, 2, 3, 5, 6 and 7 antibodies.
Figure 5. N-terminus of SENP1 and SENP2 contributes to SUMO paralog specificity
Full-length His6-SENP1 and His6-SENP2 recombinant proteins, purified by nickel resin, were labeled with HA-SUMO1 or HA-SUMO2-VS. Reactions were terminated with sample buffer and analyzed by western blotting with anti-HA antibody. Asterisks indicate probable degradation products.
Figure 6. SUMO2 specific SENPs are more abundant than SUMO1 specific SENPs in mammalian cell lysates
HA-SUMO1-VS or HA-SUMO2-VS (5ng) were reacted with HEK293, HeLa, LM TK−, A9 and Cos7 cell lysates. Reaction mixtures were analyzed by 12% SDS PAGE and western blotting with Anti-HA antibody. The expected migration for each adduct is indicated by the arrows.
Figure 7. Detection of SENPs in rabbit tissue extracts
Rabbit tissue extracts were prepared and incubated with HA-SUMO1-VS or HA-SUMO2-VS. Reactions were terminated and resolved by 12% SDS-PAGE and probed with anti-HA antibody. No SUMO-VS was added in the first lane in each panel. The arrows mark the positions of the observed SUMO-VS adducts. The asterisk marks non-specific covalent labeling with SUMO-VS.
Similar articles
- Evaluation of the activity and substrate specificity of the human SENP family of SUMO proteases.
Mendes AV, Grou CP, Azevedo JE, Pinto MP. Mendes AV, et al. Biochim Biophys Acta. 2016 Jan;1863(1):139-47. doi: 10.1016/j.bbamcr.2015.10.020. Epub 2015 Oct 30. Biochim Biophys Acta. 2016. PMID: 26522917 - Senp1 is essential for desumoylating Sumo1-modified proteins but dispensable for Sumo2 and Sumo3 deconjugation in the mouse embryo.
Sharma P, Yamada S, Lualdi M, Dasso M, Kuehn MR. Sharma P, et al. Cell Rep. 2013 May 30;3(5):1640-50. doi: 10.1016/j.celrep.2013.04.016. Epub 2013 May 16. Cell Rep. 2013. PMID: 23684609 Free PMC article. - DeSUMOylating enzymes--SENPs.
Drag M, Salvesen GS. Drag M, et al. IUBMB Life. 2008 Nov;60(11):734-42. doi: 10.1002/iub.113. IUBMB Life. 2008. PMID: 18666185 Review. - Swapping small ubiquitin-like modifier (SUMO) isoform specificity of SUMO proteases SENP6 and SENP7.
Alegre KO, Reverter D. Alegre KO, et al. J Biol Chem. 2011 Oct 14;286(41):36142-36151. doi: 10.1074/jbc.M111.268847. Epub 2011 Aug 30. J Biol Chem. 2011. PMID: 21878624 Free PMC article. - Paralogue-Specific Roles of SUMO1 and SUMO2/3 in Protein Quality Control and Associated Diseases.
Wang W, Matunis MJ. Wang W, et al. Cells. 2023 Dec 20;13(1):8. doi: 10.3390/cells13010008. Cells. 2023. PMID: 38201212 Free PMC article. Review.
Cited by
- A snapshot of the Ixodes scapularis degradome.
Mulenga A, Erikson K. Mulenga A, et al. Gene. 2011 Aug 15;482(1-2):78-93. doi: 10.1016/j.gene.2011.04.008. Epub 2011 Apr 28. Gene. 2011. PMID: 21596113 Free PMC article. - The yeast homologue of the microtubule-associated protein Lis1 interacts with the sumoylation machinery and a SUMO-targeted ubiquitin ligase.
Alonso A, D'Silva S, Rahman M, Meluh PB, Keeling J, Meednu N, Hoops HJ, Miller RK. Alonso A, et al. Mol Biol Cell. 2012 Dec;23(23):4552-66. doi: 10.1091/mbc.E12-03-0195. Epub 2012 Oct 3. Mol Biol Cell. 2012. PMID: 23034179 Free PMC article. - Function and regulation of SUMO proteases.
Hickey CM, Wilson NR, Hochstrasser M. Hickey CM, et al. Nat Rev Mol Cell Biol. 2012 Dec;13(12):755-66. doi: 10.1038/nrm3478. Nat Rev Mol Cell Biol. 2012. PMID: 23175280 Free PMC article. Review. - The chromatin modification by SUMO-2/3 but not SUMO-1 prevents the epigenetic activation of key immune-related genes during Kaposi's sarcoma associated herpesvirus reactivation.
Chang PC, Cheng CY, Campbell M, Yang YC, Hsu HW, Chang TY, Chu CH, Lee YW, Hung CL, Lai SM, Tepper CG, Hsieh WP, Wang HW, Tang CY, Wang WC, Kung HJ. Chang PC, et al. BMC Genomics. 2013 Nov 23;14(1):824. doi: 10.1186/1471-2164-14-824. BMC Genomics. 2013. PMID: 24267727 Free PMC article. - The dynamics and mechanism of SUMO chain deconjugation by SUMO-specific proteases.
Békés M, Prudden J, Srikumar T, Raught B, Boddy MN, Salvesen GS. Békés M, et al. J Biol Chem. 2011 Mar 25;286(12):10238-47. doi: 10.1074/jbc.M110.205153. Epub 2011 Jan 19. J Biol Chem. 2011. PMID: 21247896 Free PMC article.
References
- Seeler JS, Dejean A. Nuclear and unclear functions of SUMO. Nat Rev Mol Cell Biol. 2003;4:690–699. - PubMed
- Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–382. - PubMed
- Tatham MH, Jaffray E, Vaughan OA, Desterro JM, Botting CH, Naismith JH, Hay RT. Polymeric chains of SUMO-2 and SUMO-3 are conjugated to protein substrates by SAE1/SAE2 and Ubc9. J Biol Chem. 2001;276:35368–35374. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources