Trace amines depress D(2)-autoreceptor-mediated responses on midbrain dopaminergic cells - PubMed (original) (raw)
Trace amines depress D(2)-autoreceptor-mediated responses on midbrain dopaminergic cells
Ada Ledonne et al. Br J Pharmacol. 2010 Jul.
Abstract
Background and purpose: Although trace amines (TAs) are historically considered 'false neurotransmitters' on the basis of their ability to induce catecholamine release, there is evidence that they directly affect neuronal activity via TA receptors, ligand-gated receptor channels and/or sigma receptors. Here, we have investigated the effects of two TAs, tyramine (TYR) and beta-phenylethylamine (beta-PEA), on electrophysiological responses of substantia nigra pars compacta (SNpc) dopaminergic cells to the D(2) receptor agonist, quinpirole.
Experimental approach: Electrophysiological recordings of D(2) receptor-activated G-protein-gated inward rectifier K(+) channel (GIRK) currents were performed on dopaminergic cells from midbrain slices of mice and on Xenopus oocytes expressing D(2) receptors and GIRK channels.
Key results: TYR and beta-PEA reversibly reduced D(2) receptor-activated GIRK currents in a concentration-dependent manner on SNpc neurones. The inhibitory effect of TAs was still present in transgenic mice with genetically deleted TA(1) receptors and they could not be reproduced by the selective TA(1) agonist, o-phenyl-3-iodotyramine (O-PIT). Pretreatment with antagonists of sigma1 and sigma2 receptors did not block TA-induced effects. In GTPgammaS-loaded neurones, the irreversibly-activated GIRK-current was still reversibly reduced by beta-PEA. Moreover, beta-PEA did not affect basal or dopamine-evoked GIRK-currents in Xenopus oocytes.
Conclusions and implications: TAs reduced dopamine-induced responses on SNpc neurones by acting at sites different from TA(1), sigma-receptors, D(2) receptors or GIRK channels. Although their precise mechanism of action remains to be identified, TAs, by antagonizing the inhibitory effects of dopamine, may render dopaminergic neurones less sensitive to autoreceptor feedback inhibition and hence enhance their sensitivity to stimulation.
Figures
Figure 1
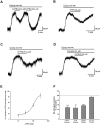
(A) Representative recordings showing the inhibitory effect of β-phenylethylamine (β-PEA) (A) and tyramine (TYR) (B) on the quinpirole-induced current. Effects were mimicked by d-amphetamine (AMPH) (C) but not by cocaine (D). Of note, cocaine does not modify the action of β-PEA. From the concentration-response curve shown in (E), the value of the IC50 was 82.9 ± 9.6 µM for β-PEA (n = 6–8 cells for each concentration). (F) The columns indicate the normalized residual quinpirole-induced current (% of control) observed in the presence of β-PEA (100 µM, n = 17, P < 0.001), TYR (100 µM, _n_ = 10, _P_ < 0.005), AMPH (30 µM, _n_ = 7, _P_ < 0.001) and cocaine (10 µM, _n_ = 4, _P_ > 0.05).
Figure 2
Effects of β-phenylethylamine (β-PEA) in the presence of enzymatic inhibition of catabolic and synthesizing pathways. (A) The concentration-response curve of β-PEA was shifted to the left in the presence of the monoamine oxidase (MAO) B inhibitor selegiline (100 nM) (n = 7 cells per concentration). (B) The L-amino acid decarboxylase (AADC) inhibitor carbidopa did not modify the effect of 300 µM β-PEA but increased the responses to 100 µM β-PEA. The enhancing effect of carbidopa at the lower concentration of β-PEA (100 µM) could be because of a drop in the endogenous level of amines caused by this AADC inhibitor and thus, a more marked effect of the exogenously applied trace amine is detected (*P < 0.05; the results depicted by the columns were obtained from n = 7 experiments).
Figure 3
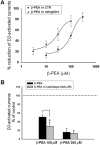
(A) (a) Representative trace showing that a consistent and reproducible inhibition of the quinpirole-induced outward current was caused by β-phenylethylamine (β-PEA; 100–300 µM) and tyramine (TYR; 100 µM) in a dopaminergic neurone obtained from a TA1 knockout mice. (b) A similar depression was caused by d-amphetamine (AMPH) (30 µM). (B) The histogram shows that the specific TA1 agonist o-phenyl-3-iodotyramine (_O_-PIT; 100 µM) had no effect on the D2 receptor-mediated outward response. In contrast to β-PEA (P < 0.001 for _O_-PIT vs. β-PEA), an infusion of _O_-PIT (100 µM) induced only a small but non-significant reduction of G protein-gated inward rectifier K+ channel (GIRK) currents (_n_ = 6, _P_ > 0.05). (C) Representative trace showing that β-PEA still inhibits the quinpirole-induced outward current in the presence of the σ2 antagonist, SM-21 (30 µM) and the σ1 antagonist, BD1047 (30 µM).
Figure 4
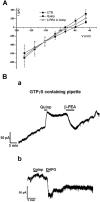
Trace amines reduced the D2 receptor-activated G protein-gated inward rectifier K+ channel (GIRK) current through a G-protein independent mechanism. (A) The current–voltage relationships performed by voltage steps in control conditions, in quinpirole and in quinpirole plus β-phenylethylamine (β-PEA; 100 µM) demonstrate that the quinpirole-induced outward currents and the β-PEA-induced inward current (quinp plus β-PEA) all reversed at –101 mV. Note that the point of intersection for the currents in the three different conditions above is close to K+ reversal potentials. Each point represents the mean ± SEM (n = 4). (B) (a) Representative current trace showing that the intracellular diffusion of GTPγS in a dopaminergic neurone caused a progressive outward current that was augmented by quinpirole (300 nM). Under this condition a relative short application of the D2 receptor agonist caused a sustained outward current that was transiently depressed by β-PEA (100 µM). (b) Noteworthy, whereas the inward current caused by β-PEA washed-out, the one induced by the mGluR 1 agonist DHPG (10 µM) was sustained indicating the involvement of G-protein in the latter case.
Figure 5
Effect of β-phenylethylamine (β-PEA) on both the basal and dopamine-evoked Kir3.2 potassium currents in heterologous expression systems. (A) Representative whole-cell current traces recorded from a Xenopus oocyte expressing Kir3.2 channels before (CTRL) and after superfusion of β-PEA (200 µM). Currents were elicited by hyperpolarizing steps to –120 mV for 2 s from a holding potential of –10 mV. The dashed line indicates zero current. (B) Time-course for the effect of β-PEA (200 µM) and Ba2+ (200 µM) on current amplitudes recorded from oocytes expressing Kir3.2 channels. The horizontal bar indicates the time of β-PEA and Ba2+ application. (C) Histogram showing the mean current amplitudes recorded at –120 mV from oocytes expressing Kir3.2 channels before and after the superfusion of β-PEA and Ba2+. (D) Representative whole-cell current traces recorded from an oocyte co-expressing Kir3.2 and D2 receptors showing the basal Kir3.2 current (CTRL), the dopamine-evoked Kir3.2 current, the effect of β-PEA (200 µM) application and wash-out (W/O). (E) Time-course of current amplitudes recorded before and after the application of dopamine (20 µM) and β-PEA (200 µM), from an oocyte co-expressing Kir3.2 and D2 receptors. The horizontal bars indicate the time of the dopamine and β-PEA application. (F) Histogram showing the mean Kir3.2 current amplitudes recorded at –120 mV under control conditions, during the application of dopamine (20 µM), β-PEA (200 µM) and after wash-out of dopamine (W/O). These results indicate that β-PEA does not affect either basal or dopamine-evoked Kir3.2 channel activity. The data are mean ± SEM of six to eight cells. (***P < 0.001, Student's _t_-test).
Similar articles
- D3 dopamine autoreceptors do not activate G-protein-gated inwardly rectifying potassium channel currents in substantia nigra dopamine neurons.
Davila V, Yan Z, Craciun LC, Logothetis D, Sulzer D. Davila V, et al. J Neurosci. 2003 Jul 2;23(13):5693-7. doi: 10.1523/JNEUROSCI.23-13-05693.2003. J Neurosci. 2003. PMID: 12843272 Free PMC article. - Tipepidine activates VTA dopamine neuron via inhibiting dopamine D₂ receptor-mediated inward rectifying K⁺ current.
Hamasaki R, Shirasaki T, Soeda F, Takahama K. Hamasaki R, et al. Neuroscience. 2013 Nov 12;252:24-34. doi: 10.1016/j.neuroscience.2013.07.044. Epub 2013 Jul 26. Neuroscience. 2013. PMID: 23896570 - Cav1.3 channels control D2-autoreceptor responses via NCS-1 in substantia nigra dopamine neurons.
Dragicevic E, Poetschke C, Duda J, Schlaudraff F, Lammel S, Schiemann J, Fauler M, Hetzel A, Watanabe M, Lujan R, Malenka RC, Striessnig J, Liss B. Dragicevic E, et al. Brain. 2014 Aug;137(Pt 8):2287-302. doi: 10.1093/brain/awu131. Epub 2014 Jun 16. Brain. 2014. PMID: 24934288 Free PMC article. - Trace amine-associated receptor 1-Family archetype or iconoclast?
Grandy DK. Grandy DK. Pharmacol Ther. 2007 Dec;116(3):355-90. doi: 10.1016/j.pharmthera.2007.06.007. Epub 2007 Jul 17. Pharmacol Ther. 2007. PMID: 17888514 Free PMC article. Review. - 2-Phenethylamines in Medicinal Chemistry: A Review.
Nieto CT, Manchado A, Belda L, Diez D, Garrido NM. Nieto CT, et al. Molecules. 2023 Jan 14;28(2):855. doi: 10.3390/molecules28020855. Molecules. 2023. PMID: 36677913 Free PMC article. Review.
Cited by
- The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity.
Miller GM. Miller GM. J Neurochem. 2011 Jan;116(2):164-76. doi: 10.1111/j.1471-4159.2010.07109.x. J Neurochem. 2011. PMID: 21073468 Free PMC article. Review. - Dopamine Evokes a Trace Amine Receptor-dependent Inward Current that is Regulated by AMP Kinase in Substantia Nigra Dopamine Neurons.
Yang W, Munhall AC, Johnson SW. Yang W, et al. Neuroscience. 2020 Feb 10;427:77-91. doi: 10.1016/j.neuroscience.2019.11.044. Epub 2019 Dec 26. Neuroscience. 2020. PMID: 31883822 Free PMC article. - TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity.
Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R, Durkin S, Zbinden KG, Norcross R, Meyer CA, Metzler V, Chaboz S, Ozmen L, Trube G, Pouzet B, Bettler B, Caron MG, Wettstein JG, Hoener MC. Revel FG, et al. Proc Natl Acad Sci U S A. 2011 May 17;108(20):8485-90. doi: 10.1073/pnas.1103029108. Epub 2011 Apr 27. Proc Natl Acad Sci U S A. 2011. PMID: 21525407 Free PMC article. - AMP-activated protein kinase slows D2 dopamine autoreceptor desensitization in substantia nigra neurons.
Yang W, Munhall AC, Johnson SW. Yang W, et al. Neuropharmacology. 2019 Nov 1;158:107705. doi: 10.1016/j.neuropharm.2019.107705. Epub 2019 Jul 10. Neuropharmacology. 2019. PMID: 31301335 Free PMC article. - Electrophysiological effects of trace amines on mesencephalic dopaminergic neurons.
Ledonne A, Berretta N, Davoli A, Rizzo GR, Bernardi G, Mercuri NB. Ledonne A, et al. Front Syst Neurosci. 2011 Jul 4;5:56. doi: 10.3389/fnsys.2011.00056. eCollection 2011. Front Syst Neurosci. 2011. PMID: 21772817 Free PMC article.
References
- Bailey BA, Philips SR, Boulton AA. In vivo release of endogenous dopamine, 5-hydroxytryptamine and some of their metabolites from rat caudate nucleus by phenylethylamine. Neurochem Res. 1987;12:173–178. - PubMed
- Barroso N, Rodriguez M. Action of β-phenylethylamine and related amines on nigrostriatal dopamine neurotransmission. Eur J Pharmacol. 1996;297:195–203. - PubMed
- Bergman J, Yasar S, Winger G. Psychomotor stimulant effects of beta-phenylethylamine in monkeys treated with MAO-B inhibitors. Psychopharmacology (Berl) 2001;159:21–30. - PubMed
- Berry MD. Mammalian central nervous system trace amines. Pharmacological amphetamines, physiological neuromodulators. J Neurochem. 2004;90:257–271. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous