Mucosal T cells in gut homeostasis and inflammation - PubMed (original) (raw)
Mucosal T cells in gut homeostasis and inflammation
Femke van Wijk et al. Expert Rev Clin Immunol. 2010 Jul.
Abstract
The antigen-rich environment of the gut interacts with a highly integrated and specialized mucosal immune system that has the challenging task of preventing invasion and the systemic spread of microbes, while avoiding excessive or unnecessary immune responses to innocuous antigens. Disruption of the mucosal barrier and/or defects in gut immune regulatory networks may lead to chronic intestinal inflammation as seen in inflammatory bowel disease. The T-cell populations of the intestine play a critical role in controlling intestinal homeostasis, and their unique phenotypes and diversities reflect the sophisticated mechanisms that have evolved to maintain the delicate balance between immune activation and tolerance at mucosal sites. In this article, we will discuss the specialized properties of mucosal T cells in the context of immune homeostasis and inflammation.
Figures
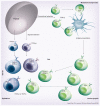
Figure 1. Intestinal T-cell subsets
The lamina propria and epithelium of the intestine harbor diverse populations of T cells. Conventional or ‘type a’ mucosal T cells that have matured in the thymus along the conventional selection pathway migrate, after antigen priming in the mesenteric lymph nodes, mainly to the lamina propria but also the epithelium. Upon entry into the epithelium, these cells often coexpress the CD8αα homodimer. Most intraepithelial lymphocytes (IELs), however, belong to two subsets of unconventional or ‘type b’ mucosal T-cell populations: the TCRγδ+ CD8αα+ IELs that are thymus derived and develop along the double-negative pathway and the TCRαβ+ CD8αα+ IELs that have matured and differentiated in the thymus along the agonist-selection pathway. Both subsets migrate as antigen-experienced directly to the intestine where the majority of cells upregulate CD8αα, while some remain double negative. DN: Double negative; TCR: T-cell receptor.
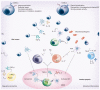
Figure 2. Mucosal T-cell regulation and activation
The functionally diverse T-cell populations of the intestine that are shaped by the gut environment are important players in sustaining the delicate immune balance between activation and regulation. The CD8αα+ IELs of the intestine play a crucial role in protecting the mucosal barrier. They are involved in maintaining and restoring barrier homeostasis by stimulating IEC turnover. Upon pathogen entry, rapid activation and high cytolytic activity of the CD8αβ+ IELs contribute to the prevention of pathogen spreading by killing infected IECs. The activation of IELs is highly controlled through the expression of inhibitory receptors that may alter the threshold for activation. In some conditions, such as celiac disease, activation of CD8αβ+ cytotoxic T lymphocytes is associated with epithelial damage. In the lamina propria (LP), all classical CD4+ Th subtypes are present. Under the influence of IECs and IELs, LP DCs acquire the ability to produce RA, thereby inducing gut-homing receptors during CD4 T-cell priming in the mLNs. The LP of the gut is enriched with both Foxp3+ Tregs and Th17 cells. Gut-derived CD103+ DCs favor the conversion of Foxp3+ iTregs in a RA- and TGF-β-mediated fashion, whereas activated DCs promote the differentiation of IL-17-producing Th17 cells via a combination of IL-6 and TGF-β. This pro- and anti-inflammatory immune deviation of iTreg and Th17 is reciprocally controlled by RA and IL-6. Specific bacteria in the small intestine have been shown to be crucial for Th17 induction, possibly through the induction of ATP release by DC. Finally, the LP is also home to agonist-selected Foxp3-expressing nTregs that, like iTregs, can produce the suppressive cytokines IL-10 and TGF-β. T helper subsets can also contribute to immune pathogenesis and damage under inflammatory conditions. Th1 and Th17 cells are implicated in Crohn’s disease, Th1 cells in celiac disease and Th2 cells in ulcerative colitis. Under these proinflammatory conditions, cytokines secreted by IECs and DCs such as IL-12, IL-23, TSLP and IL-25 promote differentiation of inflammatory Th1, Th17 and Th2 subsets, respectively. DC: Dendritic cell; IEC: Intestinal epithelial cell; IEL: Intraepithelial lymphocyte; iTreg: Induced regulatory T cell; mLN: Mesenteric lymph node; nTreg: Naturally occurring regulatory T cell; RA: Retinoic acid; TSLP: Thymic stromal lymphopoietin.
Similar articles
- A comprehensive understanding of the gut mucosal immune system in allergic inflammation.
Tokuhara D, Kurashima Y, Kamioka M, Nakayama T, Ernst P, Kiyono H. Tokuhara D, et al. Allergol Int. 2019 Jan;68(1):17-25. doi: 10.1016/j.alit.2018.09.004. Epub 2018 Oct 23. Allergol Int. 2019. PMID: 30366757 Review. - Microbiota-stimulated immune mechanisms to maintain gut homeostasis.
Chung H, Kasper DL. Chung H, et al. Curr Opin Immunol. 2010 Aug;22(4):455-60. doi: 10.1016/j.coi.2010.06.008. Epub 2010 Jul 23. Curr Opin Immunol. 2010. PMID: 20656465 Review. - Regulation of homeostasis and inflammation in the intestine.
MacDonald TT, Monteleone I, Fantini MC, Monteleone G. MacDonald TT, et al. Gastroenterology. 2011 May;140(6):1768-75. doi: 10.1053/j.gastro.2011.02.047. Gastroenterology. 2011. PMID: 21530743 Review. - Mucosal immune system of the gastrointestinal tract: maintaining balance between the good and the bad.
Ahluwalia B, Magnusson MK, Öhman L. Ahluwalia B, et al. Scand J Gastroenterol. 2017 Nov;52(11):1185-1193. doi: 10.1080/00365521.2017.1349173. Epub 2017 Jul 12. Scand J Gastroenterol. 2017. PMID: 28697651 Review. - Intestinal mucosal barrier function in health and disease.
Turner JR. Turner JR. Nat Rev Immunol. 2009 Nov;9(11):799-809. doi: 10.1038/nri2653. Nat Rev Immunol. 2009. PMID: 19855405 Review.
Cited by
- Butyrate directly decreases human gut lamina propria CD4 T cell function through histone deacetylase (HDAC) inhibition and GPR43 signaling.
Kibbie JJ, Dillon SM, Thompson TA, Purba CM, McCarter MD, Wilson CC. Kibbie JJ, et al. Immunobiology. 2021 Sep;226(5):152126. doi: 10.1016/j.imbio.2021.152126. Epub 2021 Jul 30. Immunobiology. 2021. PMID: 34365090 Free PMC article. - Pathophysiology of CD4+ T-Cell Depletion in HIV-1 and HIV-2 Infections.
Vidya Vijayan KK, Karthigeyan KP, Tripathi SP, Hanna LE. Vidya Vijayan KK, et al. Front Immunol. 2017 May 23;8:580. doi: 10.3389/fimmu.2017.00580. eCollection 2017. Front Immunol. 2017. PMID: 28588579 Free PMC article. Review. - Prenatal Stress and Ethanol Exposure: Microbiota-Induced Immune Dysregulation and Psychiatric Risks.
Camarini R, Marianno P, Hanampa-Maquera M, Oliveira SDS, Câmara NOS. Camarini R, et al. Int J Mol Sci. 2024 Sep 10;25(18):9776. doi: 10.3390/ijms25189776. Int J Mol Sci. 2024. PMID: 39337263 Free PMC article. Review. - MAP3K2 augments Th1 cell differentiation via IL-18 to promote T cell-mediated colitis.
Wu N, Chen D, Sun H, Tan J, Zhang Y, Zhang T, Han Y, Liu H, Ouyang X, Yang XD, Niu X, Zhong J, Wang Z, Su B. Wu N, et al. Sci China Life Sci. 2021 Mar;64(3):389-403. doi: 10.1007/s11427-020-1720-9. Epub 2020 Jul 28. Sci China Life Sci. 2021. PMID: 32737854 - Comparison of oral and nasal immunization with inactivated porcine epidemic diarrhea virus on intestinal immunity in piglets.
Zhang E, Wang J, Li Y, Huang L, Wang Y, Yang Q. Zhang E, et al. Exp Ther Med. 2020 Aug;20(2):1596-1606. doi: 10.3892/etm.2020.8828. Epub 2020 Jun 3. Exp Ther Med. 2020. PMID: 32742391 Free PMC article.
References
- Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat. Immunol. 2001;2(11):997–1003. - PubMed
- Iwata M, Hirakiyama A, Eshima Y, et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21(4):527–538. - PubMed
- Probert CS, Saubermann LJ, Balk S, Blumberg RS. Repertoire of the αβ T-cell receptor in the intestine. Immunol. Rev. 2007;215:215–225. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 DK054451/DK/NIDDK NIH HHS/United States
- R01 AI050265-09/AI/NIAID NIH HHS/United States
- R01 DK054451-10/DK/NIDDK NIH HHS/United States
- R01 AI064584-05/AI/NIAID NIH HHS/United States
- R01 AI064584/AI/NIAID NIH HHS/United States
- R01 AI050265/AI/NIAID NIH HHS/United States
- R01AI050265/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources