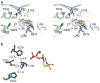P. aeruginosa PilT structures with and without nucleotide reveal a dynamic type IV pilus retraction motor - PubMed (original) (raw)
P. aeruginosa PilT structures with and without nucleotide reveal a dynamic type IV pilus retraction motor
Ana M Misic et al. J Mol Biol. 2010.
Abstract
Type IV pili are bacterial extracellular filaments that can be retracted to create force and motility. Retraction is accomplished by the motor protein PilT. Crystal structures of Pseudomonas aeruginosa PilT with and without bound beta,gamma-methyleneadenosine-5'-triphosphate have been solved at 2.6 A and 3.1 A resolution, respectively, revealing an interlocking hexamer formed by the action of a crystallographic 2-fold symmetry operator on three subunits in the asymmetric unit and held together by extensive ionic interactions. The roles of two invariant carboxylates, Asp Box motif Glu163 and Walker B motif Glu204, have been assigned to Mg(2+) binding and catalysis, respectively. The nucleotide ligands in each of the subunits in the asymmetric unit of the beta,gamma-methyleneadenosine-5'-triphosphate-bound PilT are not equally well ordered. Similarly, the three subunits in the asymmetric unit of both structures exhibit differing relative conformations of the two domains. The 12 degrees and 20 degrees domain rotations indicate motions that occur during the ATP-coupled mechanism of the disassembly of pili into membrane-localized pilin monomers. Integrating these observations, we propose a three-state "Ready, Active, Release" model for the action of PilT.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
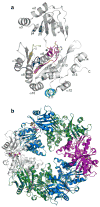
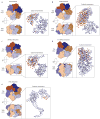
FIGURE 1. The structure of AMP-PCP bound PilT
a) AMP-PCP (yellow carbons, blue nitrogens, red oxygens, orange phosphates) is bound at the interface of the two domains, surrounded by conserved motifs of the secretion ATPases (Walker A, α6, red; Walker B, β10, blue; Asp Box, β8, yellow; His Box, β11, orange; all shown on subunit B). AMP-PCP is distant from the AIRNLIRE helix required for retraction (cyan). b) The PilT hexamer, looking down the crystallographic 2-fold axis with the N-terminal domains oriented towards the reader (subunit A, green; B, blue; C, purple; the second subunit C is grey to match the subunit shown in panel a; ligands colored as in panel a).
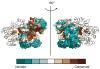
FIGURE 2. Conservation of PilT residues among secretion ATPases
Among 27 secretion ATPases, as mapped onto PilT, the most conserved residues (reddish-brown) are in the active site cleft or in proximity to the active site cleft of the neighboring subunit (grey ribbons). The least conserved residues (deep teal) are located on the periphery of the subunits. The active site ligands are colored as in Figure 1.
FIGURE 3. The nucleotide binding site and the His Box of PilT
a) Wall-eyed stereo view of the active sites of liganded (blue subunit B, green subunit A, grey Mg2+, red water) and apo (light blue subunit B, light green subunit A) PilT, aligned using the conserved RecA fold of the CTD (residues 106–301). In addition to residues discussed in the text, Arg276 coordinates the ribose while Leu109 and Leu268 sandwich the adenine moiety of the AMP-PCP. The Fo−Fc omit map was calculated from the ligand bound structure without AMP-PCP or Mg2+ ion and is contoured at 3σ. b) Thr220 and His222 (green, subunit A) and Thr132 and His229 (blue, subunit B) form a 3D His Box in the crystal structure.
FIGURE 4. Ready, Active, Release conformations of the clamp arginines of the 3 PilT subunits
a) Arginines 82 and 97 of subunit A are in a Ready conformation to bind the nucleotide. (Colors as in Fig. 1b and 3). b) The clamp arginines of subunit B are in an Active conformation, coordinating the γ-phosphate of the nucleotide. c) The clamp arginines have Released their hold on the nucleotide. d) A cartoon schematic representing the orientation of the NTDs with respect to the superimposed RecA CTDs. (Green, subunit A; blue, subunit B; purple, subunit C.)
FIGURE 5. Force generation by large domain motions among hexameric ATPase proteins
ATP binding leads to large domain movements (red arrows) within the subunit (shown in light blue cartoon). Left panels show two separate hexamer views with each subunit individually colored. The zoom in is of a single subunit (light blue) illustrating the motions of the moving domain during ATP binding, with the RecA domains fixed. Secretion ATPases (a) PilT (Chain A apo and Chain C – AMP-PCP), (b) GspE (2OAP, 2OAQ) and (c) HP0525 (1NLY, 1NLZ) exhibit domain motions that are diagonal to the central ring axis, while (d) HslU (1G3I, 1DO2) and (e) F1-ATPase (1BMF) have domain motions which are parallel and perpendicular to the ring axis, respectively.
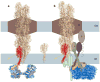
FIGURE 6. Schematic model for pilus retraction
(A) If PilT were to act on pilin directly, the NTD of PilT (blue) would contact the N-terminal tail of the bottom-most pilin subunit (red, modeled with a PilT-induced kink at proline 22) in a Type IV pilus filament (2HIL27) undergoing disassembly across the inner membrane and guided through the outer membrane by the PilQ secretin (brown). (B) More likely, inner membrane proteins are also involved in the pilus retraction pathway, and the force generated upon domain closure by PilT is transferred through an inner membrane protein to the pilin subunits, thereby providing the energy needed to disrupt hydrophobic and polar pilin:pilin interactions. The association of PilT with any particular inner membrane protein remains an unproven hypothesis. Shown are one pilus filament, one PilM, a heterodimer of PilN:PilO and one PilC (for simplicity; PilC is likely a dimer 45). Based on membrane topology predictions for the 406 residue P. aeruginosa strain PA01 inner membrane protein PilC, we have represented PilC with three full transmembrane helices and two stubby membrane-embedded reentrant helices. This prediction should be viewed with caution, as other publicly available topology analysis algorithms yield varying results.
Similar articles
- Functional role of conserved residues in the characteristic secretion NTPase motifs of the Pseudomonas aeruginosa type IV pilus motor proteins PilB, PilT and PilU.
Chiang P, Sampaleanu LM, Ayers M, Pahuta M, Howell PL, Burrows LL. Chiang P, et al. Microbiology (Reading). 2008 Jan;154(Pt 1):114-126. doi: 10.1099/mic.0.2007/011320-0. Microbiology (Reading). 2008. PMID: 18174131 - Structural and functional studies of the Pseudomonas aeruginosa minor pilin, PilE.
Nguyen Y, Harvey H, Sugiman-Marangos S, Bell SD, Buensuceso RN, Junop MS, Burrows LL. Nguyen Y, et al. J Biol Chem. 2015 Oct 30;290(44):26856-65. doi: 10.1074/jbc.M115.683334. Epub 2015 Sep 10. J Biol Chem. 2015. PMID: 26359492 Free PMC article. - The type-4 pilus is the major virulence-associated adhesin of Pseudomonas aeruginosa--a review.
Hahn HP. Hahn HP. Gene. 1997 Jun 11;192(1):99-108. doi: 10.1016/s0378-1119(97)00116-9. Gene. 1997. PMID: 9224879 Review. - Pulling together with type IV pili.
Nudleman E, Kaiser D. Nudleman E, et al. J Mol Microbiol Biotechnol. 2004;7(1-2):52-62. doi: 10.1159/000077869. J Mol Microbiol Biotechnol. 2004. PMID: 15170403 Review.
Cited by
- Minor pseudopilin self-assembly primes type II secretion pseudopilus elongation.
Cisneros DA, Bond PJ, Pugsley AP, Campos M, Francetic O. Cisneros DA, et al. EMBO J. 2012 Feb 15;31(4):1041-53. doi: 10.1038/emboj.2011.454. Epub 2011 Dec 9. EMBO J. 2012. PMID: 22157749 Free PMC article. - Core architecture of a bacterial type II secretion system.
Chernyatina AA, Low HH. Chernyatina AA, et al. Nat Commun. 2019 Nov 28;10(1):5437. doi: 10.1038/s41467-019-13301-3. Nat Commun. 2019. PMID: 31780649 Free PMC article. - Insights into FlaI functions in archaeal motor assembly and motility from structures, conformations, and genetics.
Reindl S, Ghosh A, Williams GJ, Lassak K, Neiner T, Henche AL, Albers SV, Tainer JA. Reindl S, et al. Mol Cell. 2013 Mar 28;49(6):1069-82. doi: 10.1016/j.molcel.2013.01.014. Epub 2013 Feb 14. Mol Cell. 2013. PMID: 23416110 Free PMC article. - Bidirectional pilus processing in the Tad pilus system motor CpaF.
Hohl M, Banks EJ, Manley MP, Le TBK, Low HH. Hohl M, et al. Nat Commun. 2024 Aug 5;15(1):6635. doi: 10.1038/s41467-024-50280-6. Nat Commun. 2024. PMID: 39103374 Free PMC article. - In vivo cross-linking of EpsG to EpsL suggests a role for EpsL as an ATPase-pseudopilin coupling protein in the Type II secretion system of Vibrio cholerae.
Gray MD, Bagdasarian M, Hol WG, Sandkvist M. Gray MD, et al. Mol Microbiol. 2011 Feb;79(3):786-98. doi: 10.1111/j.1365-2958.2010.07487.x. Mol Microbiol. 2011. PMID: 21255118 Free PMC article.
References
- Mattick JS. Type IV pili and twitching motility. Annu Rev Microbiol. 2002;56:289–314. - PubMed
- Varga JJ, Nguyen V, O’Brien DK, Rodgers K, Walker RA, Melville SB. Type IV pili-dependent gliding motility in the Gram-positive pathogen Clostridium perfringens and other Clostridia. Mol Microbiol. 2006;62:680–94. - PubMed
- Bradley DE. A function of Pseudomonas aeruginosa PAO polar pili: twitching motility. Can J Microbiol. 1980;26:146–54. - PubMed
- Hansen JK, Forest KT. Type IV pilin structures: insights on shared architecture, fiber assembly, receptor binding and type II secretion. J Mol Microbiol Biotechnol. 2006;11:192–207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM059721-07/GM/NIGMS NIH HHS/United States
- R01 GM059721-06A1/GM/NIGMS NIH HHS/United States
- R01 GM059721-05/GM/NIGMS NIH HHS/United States
- R01GM59721/GM/NIGMS NIH HHS/United States
- R01 GM059721-08/GM/NIGMS NIH HHS/United States
- R01 GM059721-09/GM/NIGMS NIH HHS/United States
- R01 GM059721/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases