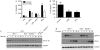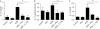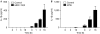Regulation of interleukin-1beta by interferon-gamma is species specific, limited by suppressor of cytokine signalling 1 and influences interleukin-17 production - PubMed (original) (raw)
Regulation of interleukin-1beta by interferon-gamma is species specific, limited by suppressor of cytokine signalling 1 and influences interleukin-17 production
Seth L Masters et al. EMBO Rep. 2010 Aug.
Abstract
Reports describing the effect of interferon-gamma (IFNgamma) on interleukin-1beta (IL-1beta) production are conflicting. We resolve this controversy by showing that IFNgamma potentiates IL-1beta release from human cells, but transiently inhibits the production of IL-1beta from mouse cells. Release from this inhibition is dependent on suppressor of cytokine signalling 1. IL-1beta and Th17 cells are pathogenic in mouse models for autoimmune disease, which use Mycobacterium tuberculosis (MTB), in which IFNgamma and IFNbeta are anti-inflammatory. We observed that these cytokines suppress IL-1beta production in response to MTB, resulting in a reduced number of IL-17-producing cells. In human cells, IFNgamma increased IL-1beta production, and this might explain why IFNgamma is detrimental for multiple sclerosis. In mice, IFNgamma decreased IL-1beta and subsequently IL-17, indicating that the adaptive immune response can provide a systemic, but transient, signal to limit inflammation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
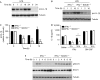
Interferon-γ inhibits interleukin-1β production by mouse macrophages. (A) Mouse macrophages were pretreated with IFNγ or saline for 2 h, then stimulated with LPS for 4 h and finally activated with MDP–TiO2 overnight to trigger IL-1β release through the inflammasome. (B) Macrophages were pretreated with IFNγ for 2 h followed by stimulation with LPS and then cells were lysed at various time points and analysed for IL-1β messenger RNA. (C) Wild-type (wt) or STAT1−/− macrophages were pretreated with IFNγ or GM-CSF for 2 h and then stimulated with LPS and MDP–TiO2 to trigger IL-1β release through the inflammasome. The inhibitory effect of IFNγ is not observed for cells deficient in STAT1. *P<0.05. GM-CSF, granulocyte–macrophage colony-stimulating factor; IFNγ, interferon-γ; IL-1β; interleukin-1β; LPS, lipopolysaccharide; MDP; muramyl dipeptide; STAT1, signal transducer and activator of transcription 1.
Figure 2
Suppressor of cytokine signalling 1 can increase interleukin-1β production by regulating the inhibitory effects of interferon-γ. (A) Mouse macrophages were pretreated with IFNγ for 1–24 h before stimulation with LPS for 4 h. Cells were then lysed and pro-IL-1β analysed by immunoblot. Pretreatment of macrophages with IFNγ alone did not induce IL-1β production. Tubulin is shown as a loading control. (B) Western blotting was performed as in (A) using IFNγ−/− (control) and IFNγ−/−SOCS1−/− macrophages. The IL-1β level returns to normal after 16 h IFNγ pretreatment in control mice, but is decreased greatly at this time point in SOCS1-deficient cells. (C) The expression levels of IL-1β relative to tubulin from three independent experiments. **P<0.01. (D) IFNγ−/− and IFNγ−/−SOCS1−/− macrophages were primed with IFNγ or GM-CSF for different times, followed by stimulation with LPS for 4 h. IL-1β release after inflammasome activation using MDP–TiO2 was decreased in SOCS1-deficient cells at the 16 h time point when quantified by ELISA. *P<0.05. (E) IFNγ−/− and IFNγ−/−SOCS1−/− macrophages were stimulated with IFNγ for 1–16 h and western blot performed for pSTAT1. STAT1 and tubulin are shown as loading controls. ELISA, enzyme-linked immunosorbent assay; GM-CSF; granulocyte–macrophage colony-stimulating factor; IFNγ, interferon-γ; IL-1β; interleukin-1β; LPS, lipopolysaccharide; MDP; muramyl dipeptide; SOCS1, suppressor of cytokine signalling 1; STAT1, signal transducer and activator of transcription 1.
Figure 3
Interferon-β inhibits interleukin-1β production in response to Mycobacterium, independent of suppressor of cytokine signalling 1. (A) Mouse DCs were pretreated with IFNγ for 3 h and stimulated with MTB overnight, LPS for 3 h, followed by MSU crystals or alum overnight. Supernatants were then analysed by ELISA for IL-1β secretion. *P<0.05. (B) Mouse DCs were pretreated with IFNγ or 100 U/ml IFNβ for 3 h, stimulated with MTB overnight and IL-1β was measured by ELISA. *P<0.05. (C) IFNγ−/− and IFNγ−/−SOCS1−/− macrophages were pretreated with IFNβ for 1–16 h, followed by LPS stimulation for 3 h. Western blot analysis was performed for pro-IL-1β showing a decrease due to IFNβ, but did not return to levels seen without IFNβ. (D) Macrophages were treated with IFNβ for the indicated times and then analysed by immunoblot using antibodies specific for pSTAT1, STAT1 and tubulin. Unlike pretreatment with IFNγ, pSTAT1 was not prolonged in SOCS1-deficient cells. DC, dendritic cell; ELISA, enzyme-linked immunosorbent assay; IFNγ, interferon-γ; IL-1β; interleukin-1β; LPS, lipopolysaccharide; MDP; muramyl dipeptide; MSU, monosodium urate; MTB, Mycobacterium tuberculosis; SOCS1, suppressor of cytokine signalling 1; STAT1, signal transducer and activator of transcription 1.
Figure 4
_Mycobacterium_-induced interleukin-1β increases interleukin-17 production, which is inhibited by interferon-γ in mouse cells. (A) Supernatants from mouse DCs pretreated with or without IFNγ for 2 h, washed and then stimulated with MTB overnight were transferred to spleen cells activated with anti-CD3 and anti-CD28 in the presence of IL-6, IL-23 and anti-IFNγ. In some cultures, the IL-1 receptor antagonist (IL-1Ra) was used to block IL-1 signalling. After 3 days, IL-17 levels were measured by ELISA. (B) Cells were cultured as in (A), then intracellular staining and FACS analysis of IL-17-producing cells were performed. (C) DCs were pretreated with IFNγ for 2 h, washed, then activated with MTB in the presence of CD4+ T cells specific for the MHC class II-restricted OVA323–339 peptide and ovalbumin peptide for 3 days. IL-17 levels in the supernatant were measured by ELISA. **P<0.01. DC, dendritic cell; ELISA, enzyme-linked immunosorbent assay; FACS, fluorescence-activated cell sorting; IFNγ, interferon-γ; IL-1β; interleukin-1β; MTB, Mycobacterium tuberculosis.
Figure 5
Interferon-γ potentiates interleukin-1β secretion from human cells. (A) Thp-1 cells were pretreated with 100 ng/ml IFNγ for different times, then stimulated with 10 ng/ml LPS for 3 h and activated with MDP–TiO2 overnight, after which the level of IL-1β was measured by ELISA. (B) Primary human dendritic cells were pretreated with IFNγ for different times, followed by stimulation with 1 μg/ml MTB and IL-1β was measured by ELISA. ELISA, enzyme-linked immunosorbent assay; IFNγ, interferon-γ; IL-1β; interleukin-1β; LPS, lipopolysaccharide; MDP; muramyl dipeptide; MTB, Mycobacterium tuberculosis.
Similar articles
- IL-21 enhances SOCS gene expression and inhibits LPS-induced cytokine production in human monocyte-derived dendritic cells.
Strengell M, Lehtonen A, Matikainen S, Julkunen I. Strengell M, et al. J Leukoc Biol. 2006 Jun;79(6):1279-85. doi: 10.1189/jlb.0905503. Epub 2006 Mar 21. J Leukoc Biol. 2006. PMID: 16551679 - The anti-inflammatory effects of interleukin-4 are not mediated by suppressor of cytokine signalling-1 (SOCS1).
Woodward EA, Prêle CM, Nicholson SE, Kolesnik TB, Hart PH. Woodward EA, et al. Immunology. 2010 Sep;131(1):118-27. doi: 10.1111/j.1365-2567.2010.03281.x. Epub 2010 Apr 6. Immunology. 2010. PMID: 20406299 Free PMC article. - Suppressor of cytokine signalling 1 (SOCS1) is a physiological regulator of the asthma response.
Lee C, Kolesnik TB, Caminschi I, Chakravorty A, Carter W, Alexander WS, Jones J, Anderson GP, Nicholson SE. Lee C, et al. Clin Exp Allergy. 2009 Jun;39(6):897-907. doi: 10.1111/j.1365-2222.2009.03217.x. Epub 2009 Mar 20. Clin Exp Allergy. 2009. PMID: 19309352 Free PMC article. - Anti-inflammatory properties of pro-inflammatory interferon-gamma.
Mühl H, Pfeilschifter J. Mühl H, et al. Int Immunopharmacol. 2003 Sep;3(9):1247-55. doi: 10.1016/S1567-5769(03)00131-0. Int Immunopharmacol. 2003. PMID: 12890422 Review. - Beyond an inflammatory mediator: Interleukin-1 in neurophysiology.
Lima TS. Lima TS. Exp Physiol. 2023 Jul;108(7):917-924. doi: 10.1113/EP090780. Epub 2023 Apr 9. Exp Physiol. 2023. PMID: 37031383 Free PMC article. Review.
Cited by
- Regulation of inflammasome signaling.
Rathinam VA, Vanaja SK, Fitzgerald KA. Rathinam VA, et al. Nat Immunol. 2012 Mar 19;13(4):333-42. doi: 10.1038/ni.2237. Nat Immunol. 2012. PMID: 22430786 Free PMC article. Review. - Suppression by thimerosal of ex-vivo CD4+ T cell response to influenza vaccine and induction of apoptosis in primary memory T cells.
Loison E, Poirier-Beaudouin B, Seffer V, Paoletti A, Abitbol V, Tartour E, Launay O, Gougeon ML. Loison E, et al. PLoS One. 2014 Apr 1;9(4):e92705. doi: 10.1371/journal.pone.0092705. eCollection 2014. PLoS One. 2014. PMID: 24690681 Free PMC article. - IL-18R-mediated HSC quiescence and MLKL-dependent cell death limit hematopoiesis during infection-induced shock.
Howard JE, Smith JNP, Fredman G, MacNamara KC. Howard JE, et al. Stem Cell Reports. 2021 Dec 14;16(12):2887-2899. doi: 10.1016/j.stemcr.2021.10.011. Epub 2021 Nov 18. Stem Cell Reports. 2021. PMID: 34798063 Free PMC article. - T-cell activation or tolerization: the Yin and Yang of bacterial superantigens.
Sähr A, Förmer S, Hildebrand D, Heeg K. Sähr A, et al. Front Microbiol. 2015 Oct 20;6:1153. doi: 10.3389/fmicb.2015.01153. eCollection 2015. Front Microbiol. 2015. PMID: 26539181 Free PMC article. - Overlapping, additive and counterregulatory effects of type II and I interferons on myeloid dendritic cell functions.
Frasca L, Lande R. Frasca L, et al. ScientificWorldJournal. 2011;11:2071-90. doi: 10.1100/2011/873895. Epub 2011 Nov 1. ScientificWorldJournal. 2011. PMID: 22125457 Free PMC article. Review.
References
- Alexander WS et al. (1999) SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell 98: 597–608 - PubMed
- Arenzana-Seisdedos F, Virelizier JL, Fiers W (1985) Interferons as macrophage-activating factors. III. Preferential effects of interferon-gamma on the interleukin 1 secretory potential of fresh or aged human monocytes. J Immunol 134: 2444–2448 - PubMed
- Boraschi D, Censini S, Tagliabue A (1984) Interferon-gamma reduces macrophage-suppressive activity by inhibiting prostaglandin E2 release and inducing interleukin 1 production. J Immunol 133: 764–768 - PubMed
- Brereton CF, Sutton CE, Lalor SJ, Lavelle EC, Mills KH (2009) Inhibition of ERK MAPK suppresses IL-23- and IL-1-driven IL-17 production and attenuates autoimmune disease. J Immunol 183: 1715–1723 - PubMed
- Brod SA, Burns DK (1994) Suppression of relapsing experimental autoimmune encephalomyelitis in the SJL/J mouse by oral administration of type I interferons. Neurology 44: 1144–1148 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources