The muscle fiber type-fiber size paradox: hypertrophy or oxidative metabolism? - PubMed (original) (raw)
Review
The muscle fiber type-fiber size paradox: hypertrophy or oxidative metabolism?
T van Wessel et al. Eur J Appl Physiol. 2010 Nov.
Abstract
An inverse relationship exists between striated muscle fiber size and its oxidative capacity. This relationship implies that muscle fibers, which are triggered to simultaneously increase their mass/strength (hypertrophy) and fatigue resistance (oxidative capacity), increase these properties (strength or fatigue resistance) to a lesser extent compared to fibers increasing either of these alone. Muscle fiber size and oxidative capacity are determined by the balance between myofibrillar protein synthesis, mitochondrial biosynthesis and degradation. New experimental data and an inventory of critical stimuli and state of activation of the signaling pathways involved in regulating contractile and metabolic protein turnover reveal: (1) higher capacity for protein synthesis in high compared to low oxidative fibers; (2) competition between signaling pathways for synthesis of myofibrillar proteins and proteins associated with oxidative metabolism; i.e., increased mitochondrial biogenesis via AMP-activated protein kinase attenuates the rate of protein synthesis; (3) relatively higher expression levels of E3-ligases and proteasome-mediated protein degradation in high oxidative fibers. These observations could explain the fiber type-fiber size paradox that despite the high capacity for protein synthesis in high oxidative fibers, these fibers remain relatively small. However, it remains challenging to understand the mechanisms by which contractile activity, mechanical loading, cellular energy status and cellular oxygen tension affect regulation of fiber size. Therefore, one needs to know the relative contribution of the signaling pathways to protein turnover in high and low oxidative fibers. The outcome and ideas presented are relevant to optimizing treatment and training in the fields of sports, cardiology, oncology, pulmonology and rehabilitation medicine.
Figures
Fig. 1
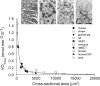
Maximum rate of oxygen consumption (_V_O2max in nmol mm−3 s−1) of muscle preparations at physiological temperature from various species plotted against the cross-sectional area (in μm2) of the muscle cells in the preparation. A hyperbola was fitted through all data points. The fit hardly deviates from the Hill-type diffusion model shown in the text and is described by the function _V_O2max = constant CSA−1. The value of the constant is calculated as the mean of the products _V_O2max and CSA for each muscle fiber type and approximates 0.4 pmol mm−1 s−1. Inset cross sections stained for succinate dehydrogenase activity. From left to right: right ventricular wall of normal rat myocardium, rat extensor digitorum longus muscle, human vastus lateralis muscle, iliofibularis muscle of Xenopus laevis; scale bar 100 μm. ratMCT right ventricular rat cardiomyocytes of a monocrotaline-induced pulmonary hypertensive rat, humanCHF vastus lateralis muscle of human chronic heart failure patients. Figure adapted from (Bekedam et al. ; Van Der Laarse et al. 1998)
Fig. 2
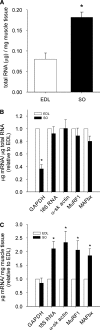
Differences in mRNA concentrations of glyceralde-3 phosphate dehydrogenase (GAPDH), 18S RNA, α-skeletal actin (α-sk actin), muscle ring finger-1 (MuRF1) and muscle atrophy F-box (MAFbx) in high oxidative rat soleus (SO) and low oxidative extensor digitorum longus (EDL) muscles (n = 6) (for methods see supplementary section I). a Total RNA (μg) normalized to muscle tissue weight (mg) showed that SO contains 2.3-fold more total RNA per milligram muscle tissue compared to EDL (p < 0.001). b mRNA normalized to total RNA relative to EDL. No differences between SO and EDL were found for any marker, except for GAPDH that showed 2.7-fold lower expression in SO (p < 0.001). c mRNA normalized to muscle tissue weight relative to EDL. GAPDH showed no significant difference between SO and EDL, whereas 18S RNA (2.1-fold), α-sk actin (2.3-fold), MuRF1 (2.1-fold) and MAFbx (1.8-fold) were all higher in SO (p < 0.05). Asterisks indicate significant difference compared to EDL. Systematic comparison of oxidative capacity and fiber cross-sectional area (CSA) from various hind limb muscles in the rat (Armstrong and Phelps 1984) show that SO predominantly (~90%) consists of slow contracting fibers with high oxidative capacity, whereas EDL contains largely (~60%) fast contracting glycolytic fibers with the lowest oxidative capacity. Within EDL, the high oxidative fibers show significantly smaller CSA than low oxidative fibers and also high oxidative fibers in SO show smaller CSA compared to the low oxidative fibers in EDL (Armstrong and Phelps ; Nakatani et al. 1999). Based on these observations and the data from Figs. 1 and 2, it can be concluded that high oxidative fibers are generally smaller and also contain more 18S RNA, α-sk actin-, MuRF1- and MAFbx-mRNA, compared to low oxidative fibers. The literature on rat SO and EDL fiber type composition does not unambiguously show that high oxidative fibers have smaller CSA compared to low oxidative fibers (Deveci et al. ; Kupa et al. ; Torrejais et al. 1999). The inconsistencies in CSA data of the latter studies compared to other studies with a more systematic approach (Armstrong and Phelps ; Nakatani et al. 1999) may be related to age, gender, muscle region or the effect of treatment. In addition, the differences in CSA between high and low oxidative fibers were not always tested for statistical significance. This impairs comparison of these studies, largely because classification of the muscle fiber type highly depends on the reaction intensity of the staining in different fiber cross sections and therefore may yield considerable variation in the estimation of fiber populations
Fig. 3
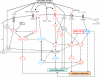
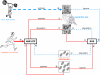
Interactions of signaling pathways and their stimuli involved in turnover of structural muscle (i.e., contractile) protein and protein associated with high oxidative metabolism. In response to contractile activity, calcium increases intracellularly through stretch-activated Ca2+ channels and from calcium stores. In addition, growth factors and cytokines are secreted in the extracellular matrix by the muscle fibers where they can bind receptors and activate signaling pathways. The type of contractile activity or mechanical loading combined with the balance between cellular energy (AMP:ATP) and oxygen levels (i.e., ROS production) determine fiber type-specific activation of pathways and thus whether contractile protein is gained or lost (for details see text). AAs amino acids, AMPK AMP-activated protein kinase, bFGF basic fibroblast growth factor, ECM extracellular matrix, FAK focal adhesion kinase, FOXO Forkhead box transcription factors O subfamily, GF + CK growth factors and cytokines, HGF hepatocyte growth factor, IL interleukin, IGF-I insulin-like growth factor-I, MAFbx muscle atrophy F-box, MAPK mitogen-activated protein kinases, MGF mechano-growth factor, mTOR mammalian target of rapamycin, MuRF muscle ring finger, NF-κB nuclear factor kappa-B, PGC-1α peroxisome proliferator-activated receptor γ coactivator-1α, PI3K phosphatidylinositol-3 kinase, PLD phospholipase D, p70S6K 70-kDa ribosomal protein S6 kinase, ROS reactive oxygen species, SC satellite cells, SR sarcoplasmatic reticulum, SRF serum response factor, TNF-α tumor necrosis factor-α, Vps34 vacuolar protein sorting mutant 34
Fig. 4
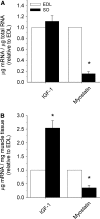
Differences in mRNA concentrations of insulin-like growth factor-I (IGF-1, all isoforms) and myostatin in rat soleus (SO) and extensor digitorum longus (EDL) muscles (n = 6) (for methods see supplementary section I). a mRNA normalized to total RNA relative to EDL. No differences between SO and EDL were found for IGF-1, but myostatin mRNA was 6.5-fold lower in SO (p < 0.001). b mRNA normalized to muscle tissue weight relative to EDL. IGF-1 expression was 2.5-fold higher in SO, whereas myostatin was 2.8-fold lower in SO (p < 0.002). Asterisks indicate significant difference compared to EDL
Fig. 5
Schematic diagram representing the regulation of muscle fiber size and oxidative metabolism. The type of contractile activity, ranging from sustained low-level activity to short high-level activity, combined with the cellular energy (AMP:ATP) and oxygen (ROS concentration) status determine the rate of synthesis and degradation of both contractile and metabolic protein. The balance between synthesis and degradation reflects the rate of protein turnover and determines whether contractile or metabolic protein is gained or lost, thereby regulating the fiber size. Solid lines are dominant processes in high oxidative fibers, whereas dashed lines are dominant in low oxidative fibers. The plus and minus signs reflect the effects of the different processes (e.g., synthesis, degradation, hypoxia) on fiber size and mitochondrial density (for details see text)
Similar articles
- Fiber hypertrophy and increased oxidative capacity can occur simultaneously in pig glycolytic skeletal muscle.
Scheffler TL, Scheffler JM, Park S, Kasten SC, Wu Y, McMillan RP, Hulver MW, Frisard MI, Gerrard DE. Scheffler TL, et al. Am J Physiol Cell Physiol. 2014 Feb 15;306(4):C354-63. doi: 10.1152/ajpcell.00002.2013. Epub 2013 Dec 4. Am J Physiol Cell Physiol. 2014. PMID: 24304835 - Cold water immersion attenuates anabolic signaling and skeletal muscle fiber hypertrophy, but not strength gain, following whole-body resistance training.
Fyfe JJ, Broatch JR, Trewin AJ, Hanson ED, Argus CK, Garnham AP, Halson SL, Polman RC, Bishop DJ, Petersen AC. Fyfe JJ, et al. J Appl Physiol (1985). 2019 Nov 1;127(5):1403-1418. doi: 10.1152/japplphysiol.00127.2019. Epub 2019 Sep 12. J Appl Physiol (1985). 2019. PMID: 31513450 - Muscle hypertrophy in prediabetic men after 16 wk of resistance training.
Stuart CA, Lee ML, South MA, Howell MEA, Stone MH. Stuart CA, et al. J Appl Physiol (1985). 2017 Oct 1;123(4):894-901. doi: 10.1152/japplphysiol.00023.2017. Epub 2017 Jun 29. J Appl Physiol (1985). 2017. PMID: 28663372 Free PMC article. - Muscle mechanics: adaptations with exercise-training.
Fitts RH, Widrick JJ. Fitts RH, et al. Exerc Sport Sci Rev. 1996;24:427-73. Exerc Sport Sci Rev. 1996. PMID: 8744258 Review. - The adaptive potential of skeletal muscle fibers.
Pette D. Pette D. Can J Appl Physiol. 2002 Aug;27(4):423-48. doi: 10.1139/h02-023. Can J Appl Physiol. 2002. PMID: 12442355 Review.
Cited by
- Impact of whole-body and skeletal muscle composition on peak oxygen uptake in heart failure: a systematic review and meta-analysis.
Schmid V, Foulkes SJ, Walesiak D, Wang J, Tomczak CR, Tucker WJ, Angadi SS, Halle M, Haykowsky MJ. Schmid V, et al. Eur Heart J Open. 2024 Sep 26;4(5):oeae082. doi: 10.1093/ehjopen/oeae082. eCollection 2024 Sep. Eur Heart J Open. 2024. PMID: 39464232 Free PMC article. - The Effect of Lower Limb Combined Neuromuscular Electrical Stimulation on Skeletal Muscle Cross-Sectional Area and Inflammatory Signaling.
Alharbi A, Li J, Womack E, Farrow M, Yarar-Fisher C. Alharbi A, et al. Int J Mol Sci. 2024 Oct 16;25(20):11095. doi: 10.3390/ijms252011095. Int J Mol Sci. 2024. PMID: 39456876 Free PMC article. - Challenging Sarcopenia: Exploring AdipoRon in Aging Skeletal Muscle as a Healthspan-Extending Shield.
Selvais CM, Davis-López de Carrizosa MA, Versele R, Dubuisson N, Noel L, Brichard SM, Abou-Samra M. Selvais CM, et al. Antioxidants (Basel). 2024 Sep 3;13(9):1073. doi: 10.3390/antiox13091073. Antioxidants (Basel). 2024. PMID: 39334732 Free PMC article. - Ability of Nicotinamide Riboside to Prevent Muscle Fatigue of Barrows Subjected to a Performance Test.
Hennesy HM, Gravely ME, Alambarrio DA, Brannen SR, McDonald JJ, Devane SA, Turner KK, Stelzleni AM, O'Quinn TG, Gonzalez JM. Hennesy HM, et al. Metabolites. 2024 Jul 31;14(8):424. doi: 10.3390/metabo14080424. Metabolites. 2024. PMID: 39195520 Free PMC article. - Effect of castration method on porcine skeletal muscle fiber traits and transcriptome profiles.
Poklukar K, Erbežnik A, Fazarinc G, Kress K, Batorek-Lukač N, Škrlep M, Stefanski V, Čandek-Potokar M, Vrecl M. Poklukar K, et al. Vet Anim Sci. 2024 Jul 26;25:100383. doi: 10.1016/j.vas.2024.100383. eCollection 2024 Sep. Vet Anim Sci. 2024. PMID: 39184227 Free PMC article.
References
- Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1 alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem. 2005;280:19587–19593. - PubMed
- Alfieri CM, Evans-Anderson HJ, Yutzey KE. Developmental regulation of the mouse IGF-I exon 1 promoter region by calcineurin activation of NFAT in skeletal muscle. Am J Physiol Cell Physiol. 2007;292:C1887–C1894. - PubMed
- Allen RE, Boxhorn LK. Regulation of skeletal-muscle satellite cell-proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor-I, and fibroblast growth-factor. J Cell Physiol. 1989;138:311–315. - PubMed
- Allen DL, Unterman TG. Regulation of myostatin expression and myoblast differentiation by FoxO and SMAD transcription factors. Am J Physiol Cell Physiol. 2007;292:C188–C199. - PubMed
- Allen DL, Monke SR, Talmadge RJ, Roy RR, Edgerton VR. Plasticity of myonuclear number in hypertrophied and atrophied mammalian skeletal-muscle fibers. J Appl Physiol. 1995;78:1969–1976. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources