TGF-beta signaling specifies axons during brain development - PubMed (original) (raw)
TGF-beta signaling specifies axons during brain development
Jason J Yi et al. Cell. 2010.
Abstract
In the mammalian brain, the specification of a single axon and multiple dendrites occurs early in the differentiation of most neuron types. Numerous intracellular signaling events for axon specification have been described in detail. However, the identity of the extracellular factor(s) that initiate neuronal polarity in vivo is unknown. Here, we report that transforming growth factor beta (TGF-beta) initiates signaling pathways both in vivo and in vitro to fate naive neurites into axons. Neocortical neurons lacking the type II TGF-beta receptor (TbetaR2) fail to initiate axons during development. Exogenous TGF-beta is sufficient to direct the rapid growth and differentiation of an axon, and genetic enhancement of receptor activity promotes the formation of multiple axons. Finally, we show that the bulk of these TGF-beta-dependent events are mediated by site-specific phosphorylation of Par6. These results define an extrinsic cue for neuronal polarity in vivo that patterns neural circuits in the developing brain.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
Figure 1
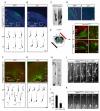
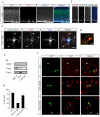
Axonal Expression of TGF-β Receptors in the Developing Mouse Neocortex (A) Sections of neocortex from embryonic day 14.5 (E14.5) mouse embryos processed for immunohistochemistry and triple labeled for TβR1, TβR2, and nestin. Scale bar, 50 μm. MZ, marginal zone; CP, cortical plate; L6, layer 6; SP, subcortical plate; IZ, intermediate zone; SVZ, subventricular zone; VZ, ventricular zone. (B) Magnified panels of area demarcated by white dashed box in (A) showing apical enrichment of TGF-β receptors in nestin-positive radial glia (arrowheads). Scale bar, 5 μm. (C) Sections of mouse neocortex at E14.5 (top) or E18 (bottom) labeled for TβR1, TβR2, and the neuron-specific β-tubulin III marker Tuj1. Arrow shows Tuj1-positive fasciculations in the IZ. Scale bar, 50 μm. Abbreviations as in (A). (D) Magnified panels of area demarcated by white dashed box in (C) showing the presence of TGF-β receptors in the IZ at E14.5. Arrowhead indicates an axon positive for both TβR1 and TβR2. Scale bar, 5 μm. (E) Sections of E14.5 mouse neocortex labeled with the corticofugal axon marker TAG1, TβR2, and the nuclear stain TOPRO-3 demonstrating the presence of TβR2 in cortical axons (arrows). Scale bar, 50 μm. (F) Magnified panels of area demarcated by white dashed box in (E) showing strong TβR2 staining in TAG1-positive axons. Scale bar, 5 μm.
Figure 2
TGF-β Signaling is Required for Neocortical Development In Vivo (A) E19.5 neocortical slice from a Tgfbr2flox/flox embryo electroporated at E14.5 with GFP to label newborn neurons. Top panel shows neuronal migration 5 days after electroporation. Bottom panel contains camera lucida traces of individual cells showing neurons with stereotypical leading edge processes and trailing edge axons (arrows). CP, cortical plate; IZ, intermediate zone; SVZ, subventricular zone; VZ, ventricular zone; DIV, days in vitro. (B) E19.5 neocortical slice from a Tgfbr2flox/flox embryo electroporated at E14.5 with GFP and Cre. Top panel shows reduced neuronal migration 5 days after electroporation. Bottom panel contains camera lucida traces of individual cells showing cells with leading edge processes but no axons (arrows). Scale bars for (A) and (B): 100 μm, top panels, 20 μm bottom panels. (C) Example images of labeled neurons from Tgfbr2flox/flox embryos expressing GFP alone (left) or GFP plus Cre (right). Arrows indicate the presence (left) or absence (right) of axons. Arrowheads indicate the leading edge dendrite. Scale bar, 15 μm. (D) Magnified panels demarcated by white boxes in (A) and (B) showing the presence (left, GFP) or absence (right, Cre) of GFP-positive axon bundles parallel to the lateral ventricles. Scale bar, 30 μm. (E) Intracranial ex vivo electroporation in E14.5 embryos. After 3 DIV, most GFP-positive neurons are found in mid-migration within the IZ (right top). At 5 DIV, many neurons have reached the CP (right bottom). Magnified panels correspond to areas demarcated by white boxes. Scale bars: 100 μm, left panels; 25 μm, right panels. (F) Organotypic neocortical slices from Tgfbr2flox/flox embryos electroporated with GFP to label newborn neurons. Top panel shows neuronal migration after 5 days. Bottom panel contains camera lucida traces of individual cells showing cells with stereotypical leading edge processes and trailing edge axons (arrows). (G) Tgfbr2flox/flox embryo electroporated to express GFP plus Cre in neuronal precursors. After 5 days, a subpopulation GFP-positive neurons reach the IZ, but many neurons fail to fully migrate (top panel). The bottom panel contains camera lucida traces showing individual cells with leading edge processes but lacking axons (arrows). Scale bars for (F) and (G): 100 μm, top panels; 20 μm, bottom panels. (H) Example images of migrating neurons from Tgfbr2flox/flox embryos expressing GFP alone (left) or GFP plus Cre (right). Arrow indicates the presence (left) or absence (right) of axons. Arrowhead indicates the leading edge dendrite. Scale bar, 22 μm. (I) Quantification of cells containing axons in wildtype (WT, GFP expressing) and TβR2-KO (TβR2-KO, GFP plus Cre) neurons. Results were pooled from 2 embryos. n = 88, 92 cells for WT and TβR2-KO, respectively. *p<0.05, Student’s t-test. (J) Time-lapse imaging of neuronal migration and polarization in E15 Tgfbr2flox/flox embryos electroporated with GFP. The arrowhead marks the soma. The arrow marks the trailing axon. Time is indicated in min:sec. Scale bar, 20 μm. See Movie S1. (K) Time-lapse imaging of neuronal migration and polarization in E15 Tgfbr2flox/flox embryos electroporated with GFP plus Cre. The arrowhead marks the soma. The arrow indicates the expected location of axon formation, which is absent. Time is indicated in min:sec. Scale bar, 20 μm. See Movie S1.
Figure 3
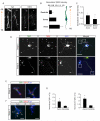
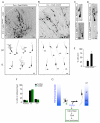
TGF-β Receptors Polarize to Nascent Axons (A) Enrichment of TβR2 in trailing-edge axons (arrows) of Tuj1-positive neurons migrating away from the ventricular zone (VZ) toward the cortical plate (CP) in an E14.5 mouse embryo. Arrowheads indicate the leading-edge dendrite. Scale bar, 20 μm. (B) Quantitative analysis of TβR2 levels along migrating neocortical neurons. Linescans were performed to measure TβR2 immunofluorescence along the leading edge dendrite, soma, and trailing edge axon, n = 12. (C) Quantitative analysis of TβR2 levels along axons, n = 12. *p<0.05, Student’s t-test. See Experimental Procedures for details. (D) Surface-labeled TβR1 and TβR2 in dissociated E18 rat hippocampal neurons show punctate distribution at the tips of all neurites (arrowheads) in stage 2 cells prior to polarization (top panels). A few hours later during axon specification, both TβR1 and TβR2 become polarized to the axon in stage 3 neurons (arrows, bottom panels) while receptor staining is reduced in dendrites (arrowheads, bottom panels). Scale bars are 15 μm for top panels and 20 μm for bottom panels. (E) Magnified panels demarcated by white boxes in the top right panel of (D) showing surface TβR1 and TβR2 staining at tips of undifferentiated stage 2 neurites. Scale bar, 3 μm. (F) Magnified panels demarcated by white boxes in the bottom right panel of (D) showing enriched TβR1 and TβR2 within the growth cone of the axon (left, arrows) and loss of surface receptor expression from the growth cone of a dendrite (right). Scale bar, 4 μm. (G) Integrated intensities of TβR1 (left) and TβR2 (right) immunofluorescence at the distal ends of axons and dendrites of stage 3 neurons. Values were normalized to the shortest neurite in each cell. n = 12 cells. * p<0.05, Student’s t-test.
Figure 4
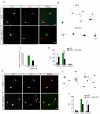
Cell Autonomous TGF-β Signaling Mediates Axon Formation (A) Dissociated hippocampal neurons from E18 rat embryos expressing GFP or GFP + TβR2-KR were fixed and stained for the axonal marker tau-1. Cells were transfected 4-6 hours after plating and fixed 65-72 hours later. Arrow indicates tau-1 positive axons, which are absent from cells expressing TβR2-KR. Scale bar, 20 μm. (B) Camera lucida traces of neurons expressing GFP (top) or GFP + TβR2-KR (bottom). Arrows indicate axons. Scale bar, 20 μm. (C) Data represent means ± SEM of the longest neurite in control cells and cells expressing TβR2-KR. n = 12, *p<0.05, Student’s t-test. (D) Quantification of axon number in cells expressing GFP alone or GFP + TβR2-KR. Data pooled from at least 3 independent experiments. GFP, n = 42; TβR2-KR, n = 53; *p<0.05, Student’s t-test. (E) Neurons expressing GFP or GFP + TβR2-WT showing multiple tau-1 positive axons emerging from cells expressing TβR2-WT (arrows). Scale bar, 20 μm. (F) Camera lucida traces of neurons expressing TβR2-WT. Arrows indicate axons. Scale bar, 20 μm. (G) Quantification of axon number in cells expressing GFP alone or GFP plus TβR2-WT. GFP, n = 41; TβR2-WT, n = 44; *p<0.05, Student’s t-test.
Figure 5
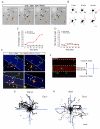
Local TGF-β is Sufficient to Specify Axon Differentiation and Growth (A) Shown is an E18 + DIV 1 hippocampal neuron undergoing selective neurite extension. The yellow circle and asterisk labels a TGF-β-conjugated bead. Black arrowheads indicate neurites untouched by beads. The red arrow indicates a neurite initially in contact with the bead. Times indicated in hours:minutes. Scale bar, 25 μm. See Movie S2. (B) Camera lucida traces of cells upon local TGF-β-induced neurite growth. Neurites initially contacting TGF-β beads (blue circles) are indicated in red. Scale bar, 25 μm. (C) Fold increase in the length of neurites either contacting (red) or not contacting (black) TGF-β beads. Data represent means ± SEM. n = 12. (D) Fold increase in the length of neurites either contacting (red) or not contacting (black) BSA beads. Data represent means ± SEM. n = 14. (E) Localized TGF-β spatially orients axon specification. Neurons were seeded on coverslips patterned with stripes of TGF-β. After 48 h, cells were fixed and stained for tau-1 and MAP2 to visualize the axon (arrow) and dendrites (arrowheads), respectively. White dashed lines indicate the borders of the TGF-β stripe. Scale bar, 50 μm. (F) For analysis, the 90 μm space between stripes was bisected (blue dashed line). Tau-1 camera lucida traces of cells seeded next to stripes were mapped onto x-y coordinates where positive y values reflect the perpendicular direction toward the TGF-β stripe and negative y values reflect the perpendicular direction away from the TGF-β stripe. Scale bar, 50 μm. (G-H) Multiple overlaid camera lucida traces of tau-1 staining from neurons grown on TGF-β (G) or BSA (H) striped coverslips. Individual cell traces shown in black. n = 45, 41. Scale bar, 25 μm.
Figure 6
TGF-β-Dependent Phosphorylation of Par6 is Required for Axon Formation (A) Co-localization of TβR1 and Par6 at the VZ (arrow) of E17 mouse neocortex. Shown is immunohistochemical triple labeling for TβR1, Par6, and the radial glial marker nestin. Scale bar, 50 μm. (B) Magnified image of the boxed region in (A) showing enriched expression of TβR1 and Par6 in the VZ. Scale bar, 5 μm. (C) Immunocytochemistry for Par6 and surface TβR1 in E18 dissociated rat hippocampal neurons. Arrows indicate colocalized puncta of Par6 and TβR1 in neurite growth cones. Scale bar, 20 μm. (D) Magnification of box in (A) showing co-localization of TβR1 clusters and Par6 in undifferentiated neurite tips. Scale bar, 2 μm. (E) Par6 and Par3 co-immunoprecipitate with TβR1 in developing brain. E18 rat forebrain lysates were subjected to immunoprecipitation with a TβR1 antibody, and co-precipitating proteins were immunoblotted for TβR1, Par6, and Par3. Input lane represents 5% of the total protein quantity used for immunoprecipitation. (F) Site-specific Par6 phosphorylation is required for axon differentiation. Hippocampal neurons were transfected with GFP, GFP-Par6-S345A, or GFP-Par6-S345E. Cells were fixed and analyzed for polarized growth. Whereas the majority of neurons expressing GFP and Par6-S345E possessed axons (arrow), neurons expressing Par6-S345A did not form distinguishable axons. Arrowhead marks the soma. Scale bar, 20 μm. (G) Data represent means ± SEM of the percent neurons with an axon. GFP, n = 38; Par6-S345A, n = 34; Par6-S345E, n = 44. *p<0.05, Student’s t-test.
Figure 7
Phosphomimetic Par6-S345E Restores Axons in TβR2 KO Neurons in Vivo (A) Neuronal progenitors in E15 Tgfbr2flox/flox embryos were electroporated with Cre plus Par6-S345A and examined five days later. The bottom panel shows representative traces of neurons in the intermediate zone (IZ) and cortical plate (CP). (B) A phosphomimetic mutant of Par6 rescues axon specification. Neuronal progenitors in E15 Tgfbr2flox/flox embryos were electroporated with Cre plus Par6-S345E. The bottom panel shows representative traces of neurons in the IZ and CP. For (A) and (B), scale bars are 50 μm for top panels and 20 μm for bottom panels. (C) Morphology of migrating TβR2-KO neurons expressing Par6-S345A, showing the absence of an axon (arrows). Scale bar, 22 μm. (D) Morphology of a migrating TβR2-KO neuron expressing Par6-S345E showing the presence of a trailing axon (arrows). Scale bar, 22 μm. (E) Data represent means ± SEM of the percent neurons with an axon. Experiments were averages from at 3-4 embryos. Par6-S345A, n = 89 cells; Par6-S345E, n = 84; *p<0.05, Student’s t-test. (F) Quantitative analysis of migration defects in TβR2-KO neuron expressing Par6-S345A or Par6-S345E. Par6-S345E did not rescue migration defects caused by the loss of TGF-β signaling. Compare to Figure S2D. Results represent experiments from at least 4 embryos. n = 2357, 4598 cells for Par6-S345A and Par6-S345E, respectively. See Supplemental Methods for details. (G) A model for TGF-β–dependent axon specification in developing neocortex. VZ, ventricular zone; IZ, intermediate zone; CP, cortical plate.
Comment in
- TGF-beta receptors PAR-ticipate in axon formation.
Dwyer ND, Winckler B. Dwyer ND, et al. Cell. 2010 Jul 9;142(1):21-3. doi: 10.1016/j.cell.2010.06.024. Cell. 2010. PMID: 20603011
Similar articles
- Dysregulated YAP1/TAZ and TGF-β signaling mediate hepatocarcinogenesis in Mob1a/1b-deficient mice.
Nishio M, Sugimachi K, Goto H, Wang J, Morikawa T, Miyachi Y, Takano Y, Hikasa H, Itoh T, Suzuki SO, Kurihara H, Aishima S, Leask A, Sasaki T, Nakano T, Nishina H, Nishikawa Y, Sekido Y, Nakao K, Shin-Ya K, Mimori K, Suzuki A. Nishio M, et al. Proc Natl Acad Sci U S A. 2016 Jan 5;113(1):E71-80. doi: 10.1073/pnas.1517188113. Epub 2015 Dec 22. Proc Natl Acad Sci U S A. 2016. PMID: 26699479 Free PMC article. - Integrin beta 1 signaling is necessary for transforming growth factor-beta activation of p38MAPK and epithelial plasticity.
Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL. Bhowmick NA, et al. J Biol Chem. 2001 Dec 14;276(50):46707-13. doi: 10.1074/jbc.M106176200. Epub 2001 Oct 5. J Biol Chem. 2001. PMID: 11590169 - Transforming growth factor-β signalling: role and consequences of Smad linker region phosphorylation.
Kamato D, Burch ML, Piva TJ, Rezaei HB, Rostam MA, Xu S, Zheng W, Little PJ, Osman N. Kamato D, et al. Cell Signal. 2013 Oct;25(10):2017-24. doi: 10.1016/j.cellsig.2013.06.001. Epub 2013 Jun 11. Cell Signal. 2013. PMID: 23770288 Review. - From receptor to nucleus: the Smad pathway.
Baker JC, Harland RM. Baker JC, et al. Curr Opin Genet Dev. 1997 Aug;7(4):467-73. doi: 10.1016/s0959-437x(97)80072-x. Curr Opin Genet Dev. 1997. PMID: 9309176 Review.
Cited by
- Hierarchical clustering of gene expression patterns in the Eomes + lineage of excitatory neurons during early neocortical development.
Cameron DA, Middleton FA, Chenn A, Olson EC. Cameron DA, et al. BMC Neurosci. 2012 Aug 1;13:90. doi: 10.1186/1471-2202-13-90. BMC Neurosci. 2012. PMID: 22852769 Free PMC article. - Atypical TGF-β signaling controls neuronal guidance in Caenorhabditis elegans.
Baltaci O, Pedersen ME, Sherry T, Handley A, Snieckute G, Cao W, Haas M, Archer S, Pocock R. Baltaci O, et al. iScience. 2022 Jan 18;25(2):103791. doi: 10.1016/j.isci.2022.103791. eCollection 2022 Feb 18. iScience. 2022. PMID: 35146399 Free PMC article. - Molecular signaling in temporomandibular joint osteoarthritis.
Lu K, Ma F, Yi D, Yu H, Tong L, Chen D. Lu K, et al. J Orthop Translat. 2021 Sep 10;32:21-27. doi: 10.1016/j.jot.2021.07.001. eCollection 2022 Jan. J Orthop Translat. 2021. PMID: 35591935 Free PMC article. Review. - Genetic variants in transforming growth factor-β gene (TGFB1) affect susceptibility to schizophrenia.
Frydecka D, Misiak B, Beszlej JA, Karabon L, Pawlak-Adamska E, Tomkiewicz A, Partyka A, Jonkisz A, Kiejna A. Frydecka D, et al. Mol Biol Rep. 2013 Oct;40(10):5607-14. doi: 10.1007/s11033-013-2662-8. Epub 2013 Sep 25. Mol Biol Rep. 2013. PMID: 24065520 Free PMC article. - Transforming growth factor β receptor signaling restrains growth of pancreatic carcinoma cells.
Zhao Z, Xi H, Xu D, Li C. Zhao Z, et al. Tumour Biol. 2015 Sep;36(10):7711-6. doi: 10.1007/s13277-015-3466-3. Epub 2015 May 3. Tumour Biol. 2015. PMID: 25934336 Retracted.
References
- Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ, Clevers HC. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 2004;116:457–466. - PubMed
- Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–342. - PubMed
- Barnes AP, Lilley BN, Pan YA, Plummer LJ, Powell AW, Raines AN, Sanes JR, Polleux F. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129:549–563. - PubMed
- Bradke F, Dotti CG. The role of local actin instability in axon formation. Science. 1999;283:1931–1934. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHMI/Howard Hughes Medical Institute/United States
- K01 MH080259/MH/NIMH NIH HHS/United States
- R01 NS047574-07/NS/NINDS NIH HHS/United States
- K01 MH080259-02/MH/NIMH NIH HHS/United States
- R01 NS047574/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases