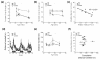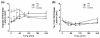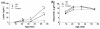Diet-induced obesity differentially regulates behavioral, biomechanical, and molecular risk factors for osteoarthritis in mice - PubMed (original) (raw)
Diet-induced obesity differentially regulates behavioral, biomechanical, and molecular risk factors for osteoarthritis in mice
Timothy M Griffin et al. Arthritis Res Ther. 2010.
Abstract
Introduction: Obesity is a major risk factor for the development of osteoarthritis in both weight-bearing and nonweight-bearing joints. The mechanisms by which obesity influences the structural or symptomatic features of osteoarthritis are not well understood, but may include systemic inflammation associated with increased adiposity. In this study, we examined biomechanical, neurobehavioral, inflammatory, and osteoarthritic changes in C57BL/6J mice fed a high-fat diet.
Methods: Female C57BL/6J mice were fed either a 10% kcal fat or a 45% kcal fat diet from 9 to 54 weeks of age. Longitudinal changes in musculoskeletal function and inflammation were compared with endpoint neurobehavioral and osteoarthritic disease states. Bivariate and multivariate analyses were conducted to determine independent associations with diet, percentage body fat, and knee osteoarthritis severity. We also examined healthy porcine cartilage explants treated with physiologic doses of leptin, alone or in combination with IL-1α and palmitic and oleic fatty acids, to determine the effects of leptin on cartilage extracellular matrix homeostasis.
Results: High susceptibility to dietary obesity was associated with increased osteoarthritic changes in the knee and impaired musculoskeletal force generation and motor function compared with controls. A high-fat diet also induced symptomatic characteristics of osteoarthritis, including hyperalgesia and anxiety-like behaviors. Controlling for the effects of diet and percentage body fat with a multivariate model revealed a significant association between knee osteoarthritis severity and serum levels of leptin, adiponectin, and IL-1α. Physiologic doses of leptin, in the presence or absence of IL-1α and fatty acids, did not substantially alter extracellular matrix homeostasis in healthy cartilage explants.
Conclusions: These results indicate that diet-induced obesity increases the risk of symptomatic features of osteoarthritis through changes in musculoskeletal function and pain-related behaviors. Furthermore, the independent association of systemic adipokine levels with knee osteoarthritis severity supports a role for adipose-associated inflammation in the molecular pathogenesis of obesity-induced osteoarthritis. Physiologic levels of leptin do not alter extracellular matrix homeostasis in healthy cartilage, suggesting that leptin may be a secondary mediator of osteoarthritis pathogenesis.
Figures
Figure 1
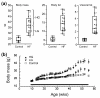
Diet-induced changes in body mass and fat levels in control and high-fat fed mice. (a) High-fat (HF)-fed mice showed much greater levels of variation in body mass, body fat, and visceral fat compared with control mice. The same individual HF mice (denoted numerically) fell in either the upper half or lower half of the bar plot distributions for these variables. Those mice in the upper half of the distribution were classified as high gainers (HG), and those in the lower half were classified as low gainers (LG). (b) Body mass in HG mice was greater than controls after 4 weeks of HF feeding compared with 37 weeks of HF feeding in LG mice (P < 0.05). Bar indicates duration of HF feeding. Data shown as mean ± standard error of the mean.
Figure 2
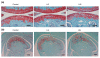
Increased osteoarthritic changes in high-fat-fed high gainer mice. (a) Representative histological images of knee joints showing increased proteoglycan depletion in high gainer (HG) mice as indicated by a loss of the red safranin-O staining. Scale bar = 100 μm. (b) Representative histological images of temporomandibular joints in control, low gainer (LG) and HG mice. There is a nonsignificant trend (P = 0.10) for increased loss of safranin-O staining in LG mice and HG mice. Scale bar = 100 μm.
Figure 3
Musculoskeletal performance in high-fat-fed mice. (a) Fore-limb grip strength reductions in high-fat (HF)-fed mice over time (three measurements/animal/timepoint). (b) Hind limb grip strength reductions in HF-fed high gainer (HG) mice over time (three measurements/animal/timepoint). (c) Knee joint osteoarthritis (OA) scores were negatively correlated with the peak vertical component of the ground reaction force (expressed per unit body mass) from the hind limb during self-selected steady-speed locomotion. (d) Spontaneous horizontal distance traveled during a 72-hour period in control and HF-fed mice at 39 weeks of age. (e) Average horizontal distance traveled during a 10-hour dark period by control and HF-fed mice at different ages. (f) Comparison of knee OA score with the cumulative dark phase distance traveled (average of 15, 20, 27, and 39 weeks of age). Data shown as mean ± standard error of the mean. *P < 0.05 versus age-matched controls.
Figure 4
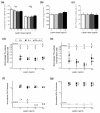
Central thermal hyperalgesia in high-fat-fed mice at 53 weeks of age. (a) Paw-withdrawal latency durations versus time for a hotplate test of centrally-mediated thermal hyperalgesia. High-fat (HF)-fed mice showed thermal hyperalgesia for the first 60 minutes of hotplate testing. (b) Tail-flick latency over time for a test of peripherally-mediated thermal hyperalgesia. HF-fed mice showed thermal hyperalgesia at 240 minutes of tail-flick testing. Data shown as mean ± standard error of the mean. *P < 0.05 for comparison with time-matched control value for either the low gainer (LG) group or the high gainer (HG) group. #P < 0.05 for time-matched HF diet versus control comparison.
Figure 5
Longitudinal serum adipokine concentrations in control and high-fat-fed mice. (a) Leptin and (b) adiponectin concentrations at 9, 22, 34, and 54 weeks of age. Data shown as mean ± standard error of the mean. *P < 0.05 versus age-matched controls. HG, high gainer; LG, low gainer.
Figure 6
Effect of physiologic leptin ± IL-1α and fatty acid on healthy cartilage extracellular matrix homeostasis. Forty-eight-hour in vitro tissue culture experiments were performed on macroscopically intact porcine femoral articular cartilage explants to determine the acute effect of leptin stimulation on extracellular matrix synthesis and degradation. (a) Effect of leptin stimulation on collagen and sulfated glycosaminoglycan (S-GAG) synthesis rates determined by radioisotope incorporation of [3H]proline and [35S]sulfate, respectively (N = 9 joints, n = 5 explants per joint). Data are normalized to the average control value per joint. (b) S-GAG release from cartilage explants due to leptin stimulation (N = 12, n = 5). (c) Nitrite and nitrate (NOx) production from cartilage explants due to leptin stimulation (N = 9, n = 5). Leptin-stimulated conditions were not significantly different from control values (P > 0.05). Effect of leptin stimulation on cartilage (d) collagen synthesis, (e) S-GAG synthesis, (f) S-GAG release, and (g) NOx production when co-treated with 0.5 mM fatty acids (FA) or 0.1 ng/ml IL-1α separately or combined (N = 3, n = 5). FA treatment was a 1:1 ratio of palmitic:oleic acids. y axes are on a log scale. *P < 0.05 versus untreated control (dashed line). #P < 0.05 versus zero leptin condition. Data shown are mean ± standard error of the mean.
Comment in
- Osteoarthritis and a high-fat diet: the full 'OA syndrome' in a small animal model.
van der Kraan PM. van der Kraan PM. Arthritis Res Ther. 2010;12(4):130. doi: 10.1186/ar3082. Epub 2010 Jul 28. Arthritis Res Ther. 2010. PMID: 20701740 Free PMC article.
Similar articles
- Correlation network analysis shows divergent effects of a long-term, high-fat diet and exercise on early stage osteoarthritis phenotypes in mice.
Griffin TM, Batushansky A, Hudson J, Lopes EBP. Griffin TM, et al. J Sport Health Sci. 2020 Mar;9(2):119-131. doi: 10.1016/j.jshs.2019.05.008. Epub 2019 May 24. J Sport Health Sci. 2020. PMID: 32099720 Free PMC article. - Systemic versus local adipokine expression differs in a combined obesity and osteoarthritis mouse model.
Hülser ML, Luo Y, Frommer K, Hasseli R, Köhler K, Diller M, Van Nie L, Rummel C, Roderfeld M, Roeb E, Schett G, Bozec A, Müller-Ladner U, Neumann E. Hülser ML, et al. Sci Rep. 2021 Aug 20;11(1):17001. doi: 10.1038/s41598-021-96545-8. Sci Rep. 2021. PMID: 34417537 Free PMC article. - Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: effects of short-term exercise.
Griffin TM, Huebner JL, Kraus VB, Yan Z, Guilak F. Griffin TM, et al. Arthritis Rheum. 2012 Feb;64(2):443-53. doi: 10.1002/art.33332. Arthritis Rheum. 2012. PMID: 21953366 Free PMC article. - Pathophysiology of obesity on knee joint homeostasis: contributions of the infrapatellar fat pad.
Santangelo KS, Radakovich LB, Fouts J, Foster MT. Santangelo KS, et al. Horm Mol Biol Clin Investig. 2016 May 1;26(2):97-108. doi: 10.1515/hmbci-2015-0067. Horm Mol Biol Clin Investig. 2016. PMID: 26812879 Review. - Systemic and Local Adipose Tissue in Knee Osteoarthritis.
Belluzzi E, El Hadi H, Granzotto M, Rossato M, Ramonda R, Macchi V, De Caro R, Vettor R, Favero M. Belluzzi E, et al. J Cell Physiol. 2017 Aug;232(8):1971-1978. doi: 10.1002/jcp.25716. Epub 2017 Mar 3. J Cell Physiol. 2017. PMID: 27925193 Review.
Cited by
- Investigating the protective effect of tanshinone IIA against chondrocyte dedifferentiation: a combined molecular biology and network pharmacology approach.
Zhang Y, Sun L, Liu X, Zhu D, Dang J, Xue Y, Fan H. Zhang Y, et al. Ann Transl Med. 2021 Feb;9(3):249. doi: 10.21037/atm-20-4023. Ann Transl Med. 2021. PMID: 33708876 Free PMC article. - Obesity aggravates the joint inflammation in a collagen-induced arthritis model through deviation to Th17 differentiation.
Jhun JY, Yoon BY, Park MK, Oh HJ, Byun JK, Lee SY, Min JK, Park SH, Kim HY, Cho ML. Jhun JY, et al. Exp Mol Med. 2012 Jul 31;44(7):424-31. doi: 10.3858/emm.2012.44.7.047. Exp Mol Med. 2012. PMID: 22513335 Free PMC article. - Obesity alters the in vivo mechanical response and biochemical properties of cartilage as measured by MRI.
Collins AT, Kulvaranon ML, Cutcliffe HC, Utturkar GM, Smith WAR, Spritzer CE, Guilak F, DeFrate LE. Collins AT, et al. Arthritis Res Ther. 2018 Oct 17;20(1):232. doi: 10.1186/s13075-018-1727-4. Arthritis Res Ther. 2018. PMID: 30333058 Free PMC article. - A commentary on modelling osteoarthritis pain in small animals.
Malfait AM, Little CB, McDougall JJ. Malfait AM, et al. Osteoarthritis Cartilage. 2013 Sep;21(9):1316-26. doi: 10.1016/j.joca.2013.06.003. Osteoarthritis Cartilage. 2013. PMID: 23973146 Free PMC article. Review. - Transgenic conversion of ω-6 to ω-3 polyunsaturated fatty acids via fat-1 reduces the severity of post-traumatic osteoarthritis.
Kimmerling KA, Oswald SJ, Huebner JL, Little D, Kraus VB, Kang JX, Wu CL, Guilak F. Kimmerling KA, et al. Arthritis Res Ther. 2020 Apr 15;22(1):83. doi: 10.1186/s13075-020-02170-7. Arthritis Res Ther. 2020. PMID: 32295649 Free PMC article.
References
- Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109:18–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG15768/AG/NIA NIH HHS/United States
- F32 AR051672-03/AR/NIAMS NIH HHS/United States
- AR48182/AR/NIAMS NIH HHS/United States
- AR51672/AR/NIAMS NIH HHS/United States
- R01 AR047442/AR/NIAMS NIH HHS/United States
- AR48852/AR/NIAMS NIH HHS/United States
- F32 AR051672/AR/NIAMS NIH HHS/United States
- EB01630/EB/NIBIB NIH HHS/United States
- AR49790/AR/NIAMS NIH HHS/United States
- AR50245/AR/NIAMS NIH HHS/United States
- P01 AR050245/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical