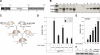Targeting RANKL to a specific subset of murine mammary epithelial cells induces ordered branching morphogenesis and alveologenesis in the absence of progesterone receptor expression - PubMed (original) (raw)
Targeting RANKL to a specific subset of murine mammary epithelial cells induces ordered branching morphogenesis and alveologenesis in the absence of progesterone receptor expression
Atish Mukherjee et al. FASEB J. 2010 Nov.
Abstract
Despite support for receptor of activated NF-κB ligand (RANKL) as a mediator of mammary progesterone action, the extent to which this cytokine can functionally contribute to established progesterone-induced mammary morphogenetic responses in the absence of other presumptive effectors is still unclear. To address this uncertainty, we developed an innovative bigenic system for the doxycycline-inducible expression of RANKL in the mammary epithelium of the progesterone receptor knockout (PRKO) mouse. In response to acute doxycycline exposure, RANKL is specifically expressed in the estrogen receptor α (ER) positive/progesterone receptor negative (ER(+)/PR(-)) cell type in the PRKO mammary epithelium, a cell type that is equivalent to the ER(+)/PR(+) cell type in the wild-type (WT) mammary epithelium. Notably, the ER(+)/PR(+) mammary cell normally expresses RANKL in the WT mammary epithelium during pregnancy. In this PRKO bigenic system, acute doxycycline-induced expression of RANKL results in ordered mammary ductal side branching and alveologenesis, morphological changes that normally occur in the parous WT mouse. This mammary epithelial expansion is accompanied by significant RANKL-induced luminal epithelial proliferation, which is driven, in part, by indirect induction of cyclin D1. Collectively, our findings support the conclusion that RANKL represents a critical mediator of mammary PR action and that restricted expression of this effector to the ER(+)/PR(+) mammary cell-type is necessary for a spatially ordered morphogenetic response to progesterone.
Figures
Figure 1.
Progesterone receptor promoter activity is restricted to ER+ cells in the PRKO mammary epithelium. A) Adult PRrtTA/rtTA/TZA bigenic females were arbitrarily treated with doxycycline for 1 mo. Doxycycline-induced β-gal activity in a longitudinal section of a PRrtTA/rtTA/TZA mammary duct is shown (black arrowhead). X-gal staining is punctate and restricted to the luminal epithelium. B) Immunohistochemical detection of ER expression in a serial section of the same tissue. Note the nonuniform expression pattern for ER in the luminal epithelial compartment (black arrowhead); a small subset of cells is also positive for ER expression in the stromal compartment (gray arrowhead). C, D) Immunofluorescence detection of β-gal (red) and ER (green) in the same mammary section from the doxycycline-treated PRrtTA/rtTA/TZA female, respectively. E) Merge of panels C and D. White arrowheads in panels C – E highlight a representative luminal epithelial cell positive for both β-gal activity and ER expression. F) Same section stained for DAPI. Blue arrowhead indicates a luminal cell negative for β-gal and ER positivity juxtaposed to a luminal cell positive for β-gal/ER immunostaining (white arrowhead). Scale bars = 100 μgmm. G) Schematic representation of the various luminal epithelial cell types in terms of ER, PR, and/or β-gal positivity in the mammary gland of the PRrtTA/rtTA/TZA bigenic and WT mouse.
Figure 2.
Generation of the PRrtTA/rtTA/TetO-RANKL bigenic mouse. A) Schematic of the TetO-RANKL transgene (_Cla_I and _Spe_I releases the full-length RANKL cDNA). B) Southern blot result that identifies the TetO-RANKL transgenic founder lines. Genomic DNA was digested with _Cla_I and _Spe_I and probed with a radioactively labeled RANKL cDNA subfragment (see Materials and Methods). Lanes 1–5 show the expected 2.3-kb hybridizing band in all 5 founder lines carrying the TetO-RANKL transgene. Because of 2 intron sequences in the endogenous murine RANKL gene, a hybridizing band of 2.3 kb is not observed in WT genomic DNA (lane 6). Lanes 7–11 represent WT genomic DNA spiked with ∼1, 5, 10, 20, and 30 copies of the full-length RANKL cDNA, respectively. C) Breeding scheme to generate the PRrtTA/+/TetO-RANKL and ultimately the PRrtTA/rtTA/TetO-RANKL mouse. D) Relative transcript levels (as measured by quantitative RT-PCR) of mammary RANKL in adult monogenic PRrtTA/+ and bigenic PRrtTA/+/TetO-RANKL mice in the absence (−) or presence (+) of doxycycline for 1 mo. Columns and error bars represent means ±
se
(_n_=5). *P < 0.05, **P < 0.001 vs. diet without doxycycline. E) Time course for doxycycline-induction of mammary RANKL transcription in the adult PRrtTA/rtTA/TetO-RANKL female Columns and error bars represent means ±
se
(_n_=5). *P < 0.05, **P < 0.001 vs. d 0 of doxycycline treatment. F) Representative Western immunoblot of doxycycline-induced mammary RANKL protein (∼45 kDa) expression in the PRrtTA/rtTA/TetO-RANKL with time. β-Actin (∼42 kDa) is a loading control.
Figure 3.
Doxycycline-induced RANKL expression elicits mammary ductal side-branching and alveolar development in the adult PRrtTA/rtTA/TetO-RANKL bigenic mouse. A) Mammary gland whole mount from an adult PRrtTA/rtTA/TetO-RANKL bigenic mouse that did not receive doxycycline. LN, lymph node (a structural reference point in the inguinal gland). B – D) Representative whole mounts of inguinal mammary glands from PRrtTA/rtTA/TetO-RANKL mouse treated with doxycycline for the time-periods indicated. B) Note the presence of nascent alveolar buds (arrowhead) following only 1 wk of doxycycline administration. C, D) With increased time of doxycycline intake, ductal side-branching and expansion of the alveolar compartment become more pronounced (arrowhead). E_–_H) Higher-magnification images of subfields shown in A_–_D, respectively. Again, note the clear evidence of ordered ductal side-branching (gray arrowhead) and lobuloalveolar development (black arrowhead) that occurs with continued doxycycline intake. Scale bars = 0.5 cm (A – D); 1 mm (E – H).
Figure 4.
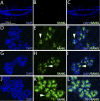
Doxycycline-induced RANKL expression in the mammary gland of the MTB/TetO-RANKL bigenic mouse induces a disorganized morphogenetic response. A, B) Whole mounts of the mammary gland from the MTB/TetO-RANKL bigenic mouse following intake of standard chow (A) or chow (and water) supplemented with doxycycline for 2 mo (B). C, D) Higher-magnification images of a subfield shown in A, B, respectively. Note the spatially disorganized ductal branching and alveologenesis in response to indiscriminate targeting of RANKL to the mammary epithelium. D) Clear evidence of hyperplasia (white arrowhead), alveolar budding (black arrowhead), and dilated or cystic ducts (gray arrowhead) are observed with doxycycline-induced RANKL expression. E, F) BrdU immunoreactivity in the mammary epithelium of the MTB/TetO-RANKL bigenic mouse after 2 mo on a standard or doxycycline-supplemented diet, respectively. E) In the absence of doxycycline, BrdU-positive cells are not detected in a transverse section of a typical duct. F) With doxycycline-induced RANKL expression, numerous BrdU-positive cells are detected in a transverse section of a lobule of alveoli (white arrowhead). Data are representative of 5 MTB/TetO-RANKL mice/treatment group.
Figure 5.
Mammary RANKL expression in the doxycycline-treated PRrtTA/rtTA/TetO-RANKL bigenic and parous wild-type mouse share a similar spatial pattern. A, B) Immunofluorescence staining for DAPI (A) and RANKL (B) in a mammary tissue section derived from the PRrtTA/rtTA/TetO-RANKL mouse without doxycycline intake. As expected, RANKL is not detected in the PRrtTA/rtTA/TetO-RANKL mammary gland in the absence of doxycycline administration. C) Merge of panels A and B. D, E) Immunofluorescence staining for DAPI (D) and RANKL (E) in a mammary section derived from the PRrtTA/rtTA/TetO-RANKL bigenic mouse administered doxycycline for 2 mo. Note the punctate spatial pattern for RANKL expression in the alveolar compartment (white arrowhead). F) Merge of panels D and E. G, H) Immunofluorescence staining for DAPI (G) and RANKL (H) in a mammary gland section from a midpregnant (d 12) wild-type mouse. Note a similar punctate staining pattern for RANKL expression in the mammary alveolar compartment of the parous mouse (compare E with H). J, K) Immunofluorescence staining for DAPI (J) and RANKL (K) expressionin the mammary gland of the similarly treated adult MTB/TetO-RANKL bitransgenic mouse. Unlike the RANKL expression pattern observed in the mammary gland of the PRrtTA/rtTA/TetO-RANKL bigenic mouse (treated with doxycycline) and of the parous WT mouse, RANKL expression is indiscriminately targeted to most mammary alveolar cells of MTB/TetO-RANKL bitransgenic. L) Merge of panels J and K. Scale bar = 100 μm.
Figure 6.
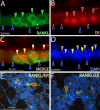
Doxycycline-induced RANKL expression in the PRrtTA/rtTA/TetO-RANKL mammary epithelium is restricted to luminal cells positive for ER expression. A) Mammary epithelium of the adult PRrtTA/rtTA/TetO-RANKL bigenic mouse (treated with doxycycline for 2 mo) stained for RANKL immunofluorescence (green). Note the presence of 4 cells that are positive for cytoplasmic RANKL expression (green arrowheads) that are interspersed with cells negative for RANKL expression (blue arrowheads). B) Same section stained for ER expression (red arrowheads). C) Merge of panels A and B. Note that RANKL and ER expression colocalize to the same cell type (yellow arrowheads). D) Same section stained for DAPI to reveal all nuclei within this field. Note that basal cells are negative for both RANKL and ER expression (brown arrowhead). E, F) Lower-magnification images of RANKL/ER expression in the alveolar compartment of the mammary gland from the doxycycline treated PRrtTA/rtTA/TetO-RANKL bigenic and WT parous mouse (d 12 postcoitum), respectively. Note the colocalization of RANKL and ER in a subset of cells (red arrowhead). Scale bars = 25 μm (A_–_D); 100 μm (E, F).
Figure 7.
RANKL elicits luminal epithelial proliferation in the mammary gland of the doxycycline-treated PRrtTA/rtTA/TetO-RANKL bigenic mouse. A, B) Transverse sections of a mammary duct stained for BrdU incorporation from the PRrtTA/rtTA/TetO-RANKL bigenic mouse in the absence or presence of doxycycline in the diet, respectively. Cells positive for BrdU incorporation are not detected in the absence of doxycycline-induced RANKL expression (A). However, a subset of cells positive for BrdU incorporation is clearly observed with doxycycline-induced RANKL expression (B; arrowhead). C) Longitudinal section of a mammary duct from the doxycycline-treated PRrtTA/rtTA/TetO-RANKL bigenic mouse showing numerous cells positive for BrdU incorporation (arrowhead). D) Histogram of the percentage of luminal epithelial cells that are positive for BrdU in the ductal (solid column) and alveolar (open column) epithelial compartments in the absence (−) or presence (+) of doxycycline (DOX) in the diet. Error bars =
se.
*P < 0.05 vs. (−) doxycycline. E) Mammary section stained for RANKL immunofluorescence from the PRrtTA/rtTA/TetO-RANKL mouse administered doxycycline for 2 mo. Note the presence of numerous RANKL-positive cells in the field (green arrowhead). F) Cells in the same field positive for BrdU incorporation (red arrowhead). G) Merge of panels E and F. Note that most RANKL-positive cells (green arrowheads) are segregated from BrdU-positive cells (red arrowheads). H) DAPI stain of the same section to reveal all nuclei in the field. I) Combined dual immunofluorescence detection of RANKL (green) and cyclin D1 (red) in the doxycycline-treated PRrtTA/rtTA/TetO-RANKL mammary gland. Note that RANKL-positive cells (green arrowhead) are segregated from cyclin D1-positive cells (red arrowhead). Scale bars = 100 μm (A–C, E–H); 50 μm (I).
Figure 8.
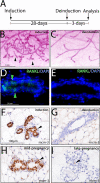
Mammary ductal side-branching and alveolar development requires continued RANKL expression. A) Schematic of the 28-d doxycycline-induction and 3-d deinduction protocol applied to adult PRrtTA/rtTA/TetO-RANKL bigenic mice prior to mammary gland whole-mount and immunohistochemical analysis. B) Typical whole mount of a mammary gland biopsied from the PRrtTA/rtTA/TetO-RANKL mouse following 28 d of doxycycline treatment. Note the presence of ductal side-branches and alveoli (arrowheads). C) Typical whole mount of the remaining mammary gland from the PRrtTA/rtTA/TetO-RANKL mouse administered doxycycline for 28 d followed by 3 d without doxycycline in the food and water (deinduction). Note the precipitous loss of ductal side-branching and alveolar budding following doxycycline withdrawal. D) Immunofluorescence detection of mammary RANKL (green arrowheads; merged with DAPI) following 28 d of doxycycline induction. E) Absence of RANKL expression following 3 d of doxycycline withdrawal. F) Significant mcm-3 expression in the mammary epithelium following 28 d of RANKL induction in the PRrtTA/rtTA/TetO-RANKL bigenic (arrowhead). G) Absence of mammary epithelial mcm-3 expression following RANKL deinduction in the PRrtTA/rtTA/TetO-RANKL bigenic. H, I) Representative immunostaining for mcm-3 expression in the mammary epithelium of the midpregnant (d 12) (H) and late-pregnant (d 18) WT mouse I.
Similar articles
- The RANKL signaling axis is sufficient to elicit ductal side-branching and alveologenesis in the mammary gland of the virgin mouse.
Fernandez-Valdivia R, Mukherjee A, Ying Y, Li J, Paquet M, DeMayo FJ, Lydon JP. Fernandez-Valdivia R, et al. Dev Biol. 2009 Apr 1;328(1):127-39. doi: 10.1016/j.ydbio.2009.01.019. Epub 2009 Jan 23. Dev Biol. 2009. PMID: 19298785 - Progesterone receptor and Stat5 signaling cross talk through RANKL in mammary epithelial cells.
Obr AE, Grimm SL, Bishop KA, Pike JW, Lydon JP, Edwards DP. Obr AE, et al. Mol Endocrinol. 2013 Nov;27(11):1808-24. doi: 10.1210/me.2013-1077. Epub 2013 Sep 6. Mol Endocrinol. 2013. PMID: 24014651 Free PMC article. - RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis.
Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, Pinkas J, Branstetter D, Dougall WC. Gonzalez-Suarez E, et al. Nature. 2010 Nov 4;468(7320):103-7. doi: 10.1038/nature09495. Epub 2010 Sep 29. Nature. 2010. PMID: 20881963 - The biology of progesterone receptor in the normal mammary gland and in breast cancer.
Obr AE, Edwards DP. Obr AE, et al. Mol Cell Endocrinol. 2012 Jun 24;357(1-2):4-17. doi: 10.1016/j.mce.2011.10.030. Epub 2011 Dec 13. Mol Cell Endocrinol. 2012. PMID: 22193050 Free PMC article. Review. - From the ranks of mammary progesterone mediators, RANKL takes the spotlight.
Fernandez-Valdivia R, Lydon JP. Fernandez-Valdivia R, et al. Mol Cell Endocrinol. 2012 Jun 24;357(1-2):91-100. doi: 10.1016/j.mce.2011.09.030. Epub 2011 Sep 22. Mol Cell Endocrinol. 2012. PMID: 21964466 Free PMC article. Review.
Cited by
- Epidermal growth factor receptor (EGFR) signaling is a key mediator of hormone-induced leukocyte infiltration in the pubertal female mammary gland.
Aupperlee MD, Zhao Y, Tan YS, Leipprandt JR, Bennett J, Haslam SZ, Schwartz RC. Aupperlee MD, et al. Endocrinology. 2014 Jun;155(6):2301-13. doi: 10.1210/en.2013-1933. Epub 2014 Apr 2. Endocrinology. 2014. PMID: 24693965 Free PMC article. - Cripto-1 ablation disrupts alveolar development in the mouse mammary gland through a progesterone receptor-mediated pathway.
Klauzinska M, McCurdy D, Rangel MC, Vaidyanath A, Castro NP, Shen MM, Gonzales M, Bertolette D, Bianco C, Callahan R, Salomon DS, Raafat A. Klauzinska M, et al. Am J Pathol. 2015 Nov;185(11):2907-22. doi: 10.1016/j.ajpath.2015.07.023. Epub 2015 Oct 1. Am J Pathol. 2015. PMID: 26429739 Free PMC article. - The Role of the RANKL/RANK Axis in the Prevention and Treatment of Breast Cancer with Immune Checkpoint Inhibitors and Anti-RANKL.
Simatou A, Sarantis P, Koustas E, Papavassiliou AG, Karamouzis MV. Simatou A, et al. Int J Mol Sci. 2020 Oct 14;21(20):7570. doi: 10.3390/ijms21207570. Int J Mol Sci. 2020. PMID: 33066388 Free PMC article. Review. - New Generation of Meso and Antiprogestins (SPRMs) into the Osteoporosis Approach.
Woźniczka M, Błaszczak-Świątkiewicz K. Woźniczka M, et al. Molecules. 2021 Oct 27;26(21):6491. doi: 10.3390/molecules26216491. Molecules. 2021. PMID: 34770897 Free PMC article. Review. - Contraceptive progestins with androgenic properties stimulate breast epithelial cell proliferation.
Shamseddin M, De Martino F, Constantin C, Scabia V, Lancelot AS, Laszlo C, Ayyannan A, Battista L, Raffoul W, Gailloud-Matthieu MC, Bucher P, Fiche M, Ambrosini G, Sflomos G, Brisken C. Shamseddin M, et al. EMBO Mol Med. 2021 Jul 7;13(7):e14314. doi: 10.15252/emmm.202114314. Epub 2021 May 27. EMBO Mol Med. 2021. PMID: 34042278 Free PMC article.
References
- Russo J., Ao X., Grill C., Russo I. H. (1999) Pattern of distribution of cells positive for estrogen receptor-α and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res. Treatment. 53, 217– 227 - PubMed
- Seagroves T. N., Lydon J. P., Hovey R. C., Vonderhaar B. K., Rosen J. M. (2000) C/EBPβ(CCAAT/enhancer binding protein) controls cell fate determination during mammary gland development. Mol. Endocrinol. 14, 359– 368 - PubMed
- Rosen J. M. (2003) Hormone receptor patterning plays a critical role in normal lobuloalveolar development and breast cancer progression. Breast Dis. 18, 3– 9 - PubMed
- Clarke R. B., Howell A., Potten C. S., Anderson E. (1997) Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 57, 4987– 4991 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials