Priming innate immune responses to infection by cyclooxygenase inhibition kills antibiotic-susceptible and -resistant bacteria - PubMed (original) (raw)
Priming innate immune responses to infection by cyclooxygenase inhibition kills antibiotic-susceptible and -resistant bacteria
Melanie J Stables et al. Blood. 2010.
Abstract
Inhibition of cyclooxygenase (COX)-derived prostaglandins (PGs) by nonsteroidal anti-inflammatory drugs (NSAIDs) mediates leukocyte killing of bacteria. However, the relative contribution of COX1 versus COX2 to this process, as well as the mechanisms controlling it in mouse and humans, are unknown. Indeed, the potential of NSAIDs to facilitate leukocyte killing of drug-resistant bacteria warrants investigation. Therefore, we carried out a series of experiments in mice and humans, finding that COX1 is the predominant isoform active in PG synthesis during infection and that its prophylactic or therapeutic inhibition primes leukocytes to kill bacteria by increasing phagocytic uptake and reactive oxygen intermediate-mediated killing in a cyclic adenosine monophosphate (cAMP)-dependent manner. Moreover, NSAIDs enhance bacterial killing in humans, exerting an additive effect when used in combination with antibiotics. Finally, NSAIDs, through the inhibition of COX prime the innate immune system to mediate bacterial clearance of penicillin-resistant Streptococcus pneumoniae serotype 19A, a well-recognized vaccine escape serotype of particular concern given its increasing prevalence and multi-antibiotic resistance. Therefore, these data underline the importance of lipid mediators in host responses to infection and the potential of inhibitors of PG signaling pathways as adjunctive therapies, particularly in the con-text of antibiotic resistance.
Conflict of interest statement
No author has conflicting financial interest.
Figures
Figure 1
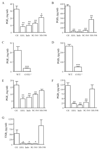
Characterisation of infectious peritonitis. Injecting GBS i.p. to W/T mice triggers an immediate (A) leukocytes infiltrate comprising (B) GR1-positive PMNs and F4/80-positive macrophages as well as (C) CD3-positive T and CD19-positive B cells. Total peritoneal leukocytes were prepared for (D) COX 1 and COX 2 protein expression while (E) PGE2, (F) prostacyclin, measured as its stable metabolites 6-keto PGF1α and 2,3-dinor 6-keto PGF1α, (G) TNFα and (H) IL-10 were determined in the cell-free inflammatory exudates. Values are expressed as the mean ± SEM of 5-12 mice/group.
Figure 2
COX 1 is the predominant isoforms functional during infectious peritonitis. Selective COX inhibitors (SC-560 for COX 1 and NS-398 for COX 2) and non-selective COX inhibitors (aspirin and indomethacin) were dosed orally to W/T animals 1h before GBS injection. Cell-free peritoneal exudate levels of (A) PGE2 and (B) PGD2 were measured 3h after GBS with similar results obtained for (C) PGE2 and (D) PGD2 using COX 1 knockout mice. In addition, COX inhibitors were dosed to mice 1h before i.p. zymosan (non-infectious stimulus) with cell-free peritoneal exudate levels of (E) PGE2 and (F) PGD2 measured 3h later. NS-398 was used at dosing levels that do not inhibit (G) COX 1-derived plasma TxB2 measured 3h after i.p. GBS injection. Data were analysed by one-way ANOVA and Dunnett’s multiple comparison test or by unpaired Student T-test. Values are expressed as the mean ± SEM of between 5-12 mice/group. * P < 0.05; ** P < 0.01 and *** P <0.001.
Figure 3
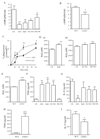
COX inhibition enhances bacteria killing in rodents. Mice were given dual (indomethacin/aspirin), COX 1 (SC-560) or COX 2 (NS-398) selective inhibitors orally either (A) 1h before or (B) therapeutically 1h after i.p. inoculation of GBS. To exclude dampened bacterial translocation as a potential explanation for reduced plasma GBS titres following COX inhibition, peritoneal exudate levels of (C) GBS were measured 3h after inoculation. GBS was also injected i.p. to (D) COX 1 knockout mice with plasma taken 3h later for overnight cfu culture number determination as well as (E) incubated in culture media only with NSAIDs in the absence of leukocytes. Data were analysed by one-way ANOVA and Dunnett’s multiple comparison test or by unpaired Student T-test. Values are expressed as the mean ± SEM of between 5-12 mice/group. * P < 0.05 and ** P < 0.01.
Figure 4
COX inhibition alters phagocytosis, cytokine synthesis and bacterial killing mechanisms in a cAMP-dependent manner. GBS was injected i.p. to (A) W/T mice dosed orally 1h earlier with indomethacin (dual COX inhibitor), SC-560 (COX 1 inhibitor) or NS-398 (COX 2 inhibitor) as well as to (B) COX 1 knockout mice. 3h after GBS injection (A-B) cAMP was measured in cell-free exudates while (C) phagocytosis and (D-E) NADPH oxidase activity was determined in total leukocytes. Cell-free exudate levels of (F-G) TNFα and IL-10 were also determined in NSAID-treated W/Ts and (H-I) COX 1 knockouts. This COX-inhibited differential change in W/T mice (J) bacterial killing, (K) NADPH oxidase activity and (L-M) cytokine synthesis was reversed by 15(S)-15 methyl PGE2 (EP agonist) or db-cAMP given 5mins before GBS and therefore 55mins after NSAIDs. Data is represented and analysed by an unpaired Student’s T-test or ANOVA followed by either Dunnett’s or Bonferroni multiple comparison tests. Values are expressed as the mean ± SEM of 5-12 mice/group. * P < 0.05 and ** P < 0.01. *** P < 0.001.
Figure 4
COX inhibition alters phagocytosis, cytokine synthesis and bacterial killing mechanisms in a cAMP-dependent manner. GBS was injected i.p. to (A) W/T mice dosed orally 1h earlier with indomethacin (dual COX inhibitor), SC-560 (COX 1 inhibitor) or NS-398 (COX 2 inhibitor) as well as to (B) COX 1 knockout mice. 3h after GBS injection (A-B) cAMP was measured in cell-free exudates while (C) phagocytosis and (D-E) NADPH oxidase activity was determined in total leukocytes. Cell-free exudate levels of (F-G) TNFα and IL-10 were also determined in NSAID-treated W/Ts and (H-I) COX 1 knockouts. This COX-inhibited differential change in W/T mice (J) bacterial killing, (K) NADPH oxidase activity and (L-M) cytokine synthesis was reversed by 15(S)-15 methyl PGE2 (EP agonist) or db-cAMP given 5mins before GBS and therefore 55mins after NSAIDs. Data is represented and analysed by an unpaired Student’s T-test or ANOVA followed by either Dunnett’s or Bonferroni multiple comparison tests. Values are expressed as the mean ± SEM of 5-12 mice/group. * P < 0.05 and ** P < 0.01. *** P < 0.001.
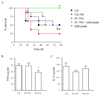
Figure 5
Prophylactic but not therapeutic COX inhibition triggers pro-inflammatory cytokine synthesis. W/T mice were injected i.p. with GBS 1h after oral administration of NS-398 (COX 2 inhibitor), SC-560 (COX 1 inhibitor) or SC-560 plus infliximab with (A) complete reversal of COX 1-mediated cytokine storm (TNFα synthesis, Figure 4F) afforded by infliximab. SC-560 and NS-398 were dosed orally 1h after GBS injection causing little effects on (B-C) inflammatory cytokine levels in cell-free inflammatory exudates 3h later. Survival analysis was completed on 8 mice/group and the log-rank test was used to compare each group against one another. ** values represent P < 0.01 for controls versus Sc-560/infliximab. For cytokines, values are expressed as the mean ± SEM of 5-7 mice/group and analysed by one-way ANOVA and Dunnett’s multiple comparison test. *values represent P < 0.05. Survival experiments had 8 mice/group.
Figure 6
COX inhibition primes human whole blood to kill bacteria. Peripheral blood of 8 healthy male volunteers between the ages of 25-50 was taken before and 1h after ingestion of 500mg naproxen and incubated ex vivo with opsonised GBS. After 60min aliquots were taken to determine (A i) numbers of viable bacteria determined by overnight incubation on agar plates or (A ii) leukocyte NADPH oxidase activity. Blood from volunteers taken naproxen was further incubated with (B i-iii) penicillin (0.075μg/ml) to determine NSAID inhibitory/additive effects on bacterial killing with antibiotics. (C) Blood was also treated 400μM naproxen for 30min and stimulated with FAM-SE labelled S. pneumoniae (ST193) 5min to determine bacterial phagocytosis and to show that (D) NSAIDs do not interfere with opsinisation of bacteria. Data were analysed by paired Student’s T-test. * values represent P < 0.05 and ** values represent P < 0.01.
Figure 7
Inhibiting PG signalling primes human whole blood to kill antibiotic-resistant bacteria. Healthy human peripheral blood was pre-incubated with/without penicillin and a pan EP/DP PG receptor antagonist targeted against EP1, EP2, EP3-III, and DP1 receptors as well as against individual PG receptors including EP4, DP1 and IP. Whole blood was then stimulated with either (A) serum-opsonised penicillin-susceptible (ST193) or (B) intermediate penicillin-resistant (ST199) S. pneumonia for 1h. After which time, numbers of surviving S. pneumoniae (cfu) were quantified 24h later on agar plates. Data were analysed by one-way ANOVA and Bonferroni’s multiple comparison test. * values represent P < 0.05, ** values P < 0.01 and *** values P < 0.001.
Comment in
- Another miracle left in aspirin?
Aronoff DM. Aronoff DM. Blood. 2010 Oct 21;116(16):2866-7. doi: 10.1182/blood-2010-07-296756. Blood. 2010. PMID: 20966173 No abstract available.
Similar articles
- Dual acting anti-inflammatory drugs: a reappraisal.
Bertolini A, Ottani A, Sandrini M. Bertolini A, et al. Pharmacol Res. 2001 Dec;44(6):437-50. doi: 10.1006/phrs.2001.0872. Pharmacol Res. 2001. PMID: 11735348 Review. - Cyclooxygenase-1 and -2 Play Contrasting Roles in _Listeria_-Stimulated Immunity.
Theisen E, McDougal CE, Nakanishi M, Stevenson DM, Amador-Noguez D, Rosenberg DW, Knoll LJ, Sauer JD. Theisen E, et al. J Immunol. 2018 Jun 1;200(11):3729-3738. doi: 10.4049/jimmunol.1700701. Epub 2018 Apr 20. J Immunol. 2018. PMID: 29678951 Free PMC article. - Therapeutic Synergy Between Antibiotics and Pulmonary Toll-Like Receptor 5 Stimulation in Antibiotic-Sensitive or -Resistant Pneumonia.
Matarazzo L, Casilag F, Porte R, Wallet F, Cayet D, Faveeuw C, Carnoy C, Sirard JC. Matarazzo L, et al. Front Immunol. 2019 Apr 9;10:723. doi: 10.3389/fimmu.2019.00723. eCollection 2019. Front Immunol. 2019. PMID: 31024555 Free PMC article. - Selective COX-2 inhibitors and dual acting anti-inflammatory drugs: critical remarks.
Bertolini A, Ottani A, Sandrini M. Bertolini A, et al. Curr Med Chem. 2002 May;9(10):1033-43. doi: 10.2174/0929867024606650. Curr Med Chem. 2002. PMID: 12733982 Review. - Prostaglandins and cyclooxygenases [correction of cycloxygenases] in the spinal cord.
Vanegas H, Schaible HG. Vanegas H, et al. Prog Neurobiol. 2001 Jul;64(4):327-63. doi: 10.1016/s0301-0082(00)00063-0. Prog Neurobiol. 2001. PMID: 11275357 Review.
Cited by
- Randomised clinical study: oral aspirin 325 mg daily vs placebo alters gut microbial composition and bacterial taxa associated with colorectal cancer risk.
Prizment AE, Staley C, Onyeaghala GC, Vivek S, Thyagarajan B, Straka RJ, Demmer RT, Knights D, Meyer KA, Shaukat A, Sadowsky MJ, Church TR. Prizment AE, et al. Aliment Pharmacol Ther. 2020 Sep;52(6):976-987. doi: 10.1111/apt.16013. Epub 2020 Aug 8. Aliment Pharmacol Ther. 2020. PMID: 32770859 Free PMC article. Clinical Trial. - Postpartum group a Streptococcus sepsis and maternal immunology.
Mason KL, Aronoff DM. Mason KL, et al. Am J Reprod Immunol. 2012 Feb;67(2):91-100. doi: 10.1111/j.1600-0897.2011.01083.x. Epub 2011 Oct 24. Am J Reprod Immunol. 2012. PMID: 22023345 Free PMC article. Review. - The local inflammatory responses to infection of the peritoneal cavity in humans: their regulation by cytokines, macrophages, and other leukocytes.
Fieren MW. Fieren MW. Mediators Inflamm. 2012;2012:976241. doi: 10.1155/2012/976241. Epub 2012 Feb 26. Mediators Inflamm. 2012. PMID: 22481867 Free PMC article. Review. - The resolution of inflammation.
Buckley CD, Gilroy DW, Serhan CN, Stockinger B, Tak PP. Buckley CD, et al. Nat Rev Immunol. 2013 Jan;13(1):59-66. doi: 10.1038/nri3362. Epub 2012 Nov 30. Nat Rev Immunol. 2013. PMID: 23197111 Review. - The roles of injury and nonsteroidal anti-inflammatory drugs in the development and outcomes of severe group A streptococcal soft tissue infections.
Bryant AE, Bayer CR, Aldape MJ, Stevens DL. Bryant AE, et al. Curr Opin Infect Dis. 2015 Jun;28(3):231-9. doi: 10.1097/QCO.0000000000000160. Curr Opin Infect Dis. 2015. PMID: 25918957 Free PMC article. Review.
References
- Livermore DM. Minimising antibiotic resistance. Lancet Infect Dis. 2005;5(7):450–459. - PubMed
- Moncada S, Vane JR. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol Rev. 1978;30(3):293–331. - PubMed
- Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56(3):387–437. - PubMed
- Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M. Cutting edge: macrophage inhibition by cyclic AMP (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J Immunol. 2005;174(2):595–599. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials