Male-like sexual behavior of female mouse lacking fucose mutarotase - PubMed (original) (raw)
Male-like sexual behavior of female mouse lacking fucose mutarotase
Dongkyu Park et al. BMC Genet. 2010.
Abstract
Background: Mutarotases are recently characterized family of enzymes that are involved in the anomeric conversions of monosaccharides. The mammalian fucose mutarotase (FucM) was reported in cultured cells to facilitate fucose utilization and incorporation into protein by glycosylation. However, the role of this enzyme in animal has not been elucidated.
Results: We generated a mutant mouse specifically lacking the fucose mutarotase (FucM) gene. The FucM knockout mice displayed an abnormal sexual receptivity with a drastic reduction in lordosis score, although the animals were fertile due to a rare and forced intromission by a typical male. We examined the anteroventral periventricular nucleus (AVPv) of the preoptic region in brain and found that the mutant females showed a reduction in tyrosine hydoxylase positive neurons compared to that of a normal female. Furthermore, the mutant females exhibited a masculine behavior, such as mounting to a normal female partner as well as showing a preference to female urine. We found a reduction of fucosylated serum alpha-fetoprotein (AFP) in a mutant embryo relative to that of a wild-type embryo.
Conclusions: The observation that FucM-/- female mouse exhibits a phenotypic similarity to a wild-type male in terms of its sexual behavior appears to be due to the neurodevelopmental changes in preoptic area of mutant brain resembling a wild-type male. Since the previous studies indicate that AFP plays a role in titrating estradiol that are required to consolidate sexual preference of female mice, we speculate that the reduced level of AFP in FucM-/- mouse, presumably resulting from the reduced fucosylation, is responsible for the male-like sexual behavior observed in the FucM knock-out mouse.
Figures
Figure 1
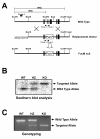
Targeted Disruption of the FucM Gene. (A) Schematic diagram for the targeted disruption of the FucM gene. The structures of the targeting vector, wild-type, and disrupted FucM alleles are shown. The solid boxes represent the exons (a total of six in the wild-type). The restriction sites used for constructing the targeting vector and for analysis by Southern blot are EcoRV, BssHI, ScaI, and KpnI. A puromycin expressing cassette was inserted between the EcoRV and ScaI sites, eliminating exons 2-4 and the EcoRV site of the WT gene. (B) Southern analysis for genomic DNA from the wild-type (+/+), heterozygous (+/-) and FucM null (-/-) mice. The expected sizes of the wild-type and disrupted FucM alleles are 10 and 16 Kb, respectively, which were detected by Southern blotting with EcoRV digestion and hybridization using the probe 1 shown in (A). (C) The wild-type (primer 1 and 2) and disrupted alleles (primer 3 and 4) were also detected by PCR.
Figure 2
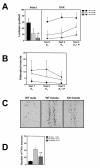
Sexual behaviors of wild-type and FucM knockout females. (A) Lordosis quotients of gonads-intact (left) and ovariectomized females (right). Lordotic responses by three consecutive tests were observed for the ovariectomized wild-type controls and FucM gene-disrupted mice after treatments with E2 (test 1 and 2) and E2 plus progesterone (test 3). The tests were carried out at a one-week interval. The scores of the KO females were different with P < 0.001 (*) from that of the wild-type and _P_ > 0.05 (#) from that of heterozygote. (B) Frequencies of attempted mounts, mounts and intromission-like behaviors in three consecutive tests of subject females with a female partner. (C) Photomicrographs of sections in AVPv stained by immunohistochemistry for tyrosine hydroxylase in males, wild-type females, and FucM KO females. (D) Numbers of tyrosine hydroxylase-immunoreactive (ir) neurons in the sections shown in C: *, P < 0.001 compared to that of the wild-type males; #, P < 0.001 compared to that of wild-type females. Data are shown as mean ± s.e.m.
Figure 3
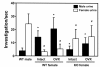
The olfactory response of female mice lacking FucM. The mean time spent sniffing male and female urine by wild-type males (n = 15), wild-type females (intact, n = 15; OVX, n = 13) and FucM KO females (intact, n = 12; OVX, n = 10). Result of post-hoc comparisons by the Fisher PLSD test are indicated as follows: *, P < 0.001 when compared to wild-type males for male urine; #, P < 0.001 when compared to wild-type males for female urine. Data are expressed as mean ± s.e.m.
Figure 4
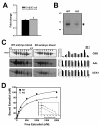
Amounts of AFP and its binding to 17β-estradiol in embryonic sera. (A) Gene expression analysis of AFP in WT and KO embryos at E16.5. The amplified copies of AFP were normalized against GAPDH, from which the ratio between copy numbers was determined to obtain normalized fold change. (B) Fetal mouse sera containing AFP (arrow) were visualized by 10% SDS-PAGE, followed by Coomassie brilliant blue (CBB) staining, which were obtained from 25 each of wild-type and mutant embryos at E16.5. They were diluted 1/30 and loaded. (C) Embryonic serum proteins from the WT and the FucM deficient mice were subjected to 2-D electrophoresis, followed by CBB staining. After blotting onto a nitrocellulose membrane, lectin blotting was performed. Aleuria aurantia lectin (AAL) binds to N_-linked oligosaccharides with L-fucose linked (α-1,3/α-1,6) to the proximal GlcNAc residue, while Ulex europaeus agglutinin I preferentially binds to Fuc_α(1-2)Gal_β_(1-4)GlcNAc. Spot intensities were analyzed using ImageJ to estimate relative fucosylation levels of AFP. (D) Scatchard analyses for the interactions of 17β-estradiol (E2) with mouse embryonic sera are shown, which were determined by the dextran-coated charcoal (DCC) technique as described in the Methods section.
Similar articles
- Characterization and role of fucose mutarotase in mammalian cells.
Park D, Ryu KS, Choi D, Kwak J, Park C. Park D, et al. Glycobiology. 2007 Sep;17(9):955-62. doi: 10.1093/glycob/cwm066. Epub 2007 Jun 29. Glycobiology. 2007. PMID: 17602138 - The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior.
Kauffman AS, Park JH, McPhie-Lalmansingh AA, Gottsch ML, Bodo C, Hohmann JG, Pavlova MN, Rohde AD, Clifton DK, Steiner RA, Rissman EF. Kauffman AS, et al. J Neurosci. 2007 Aug 15;27(33):8826-35. doi: 10.1523/JNEUROSCI.2099-07.2007. J Neurosci. 2007. PMID: 17699664 Free PMC article. - The Role of Kisspeptin in Sexual Behavior.
Hellier V, Brock O, Bakker J. Hellier V, et al. Semin Reprod Med. 2019 Mar;37(2):84-92. doi: 10.1055/s-0039-3400992. Epub 2019 Dec 17. Semin Reprod Med. 2019. PMID: 31847028 Review. - The sexually dimorphic medial preoptic nucleus of quail: a key brain area mediating steroid action on male sexual behavior.
Panzica GC, Viglietti-Panzica C, Balthazart J. Panzica GC, et al. Front Neuroendocrinol. 1996 Jan;17(1):51-125. doi: 10.1006/frne.1996.0002. Front Neuroendocrinol. 1996. PMID: 8788569 Review.
Cited by
- Fucose Ameliorates Tryptophan Metabolism and Behavioral Abnormalities in a Mouse Model of Chronic Colitis.
Borisova MA, Snytnikova OA, Litvinova EA, Achasova KM, Babochkina TI, Pindyurin AV, Tsentalovich YP, Kozhevnikova EN. Borisova MA, et al. Nutrients. 2020 Feb 11;12(2):445. doi: 10.3390/nu12020445. Nutrients. 2020. PMID: 32053891 Free PMC article. - Regional gene expression patterns are associated with task-specific brain activation during reward and emotion processing measured with functional MRI.
Komorowski A, Murgaš M, Vidal R, Singh A, Gryglewski G, Kasper S, Wiltfang J, Lanzenberger R, Goya-Maldonado R. Komorowski A, et al. Hum Brain Mapp. 2022 Dec 1;43(17):5266-5280. doi: 10.1002/hbm.26001. Epub 2022 Jul 7. Hum Brain Mapp. 2022. PMID: 35796185 Free PMC article. - Myc suppresses male-male courtship in Drosophila.
Pan Y, Li W, Deng Z, Sun Y, Ma X, Liang R, Guo X, Sun Y, Li W, Jiao R, Xue L. Pan Y, et al. EMBO J. 2022 Apr 4;41(7):e109905. doi: 10.15252/embj.2021109905. Epub 2022 Feb 15. EMBO J. 2022. PMID: 35167135 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases