Increased activity and expression of histone deacetylase 1 in relation to tumor necrosis factor-alpha in synovial tissue of rheumatoid arthritis - PubMed (original) (raw)
Increased activity and expression of histone deacetylase 1 in relation to tumor necrosis factor-alpha in synovial tissue of rheumatoid arthritis
Tomoko Kawabata et al. Arthritis Res Ther. 2010.
Abstract
Introduction: The purpose of this study was to investigate the profile of histone deacetylase (HDAC) expression in the synovial tissue of rheumatoid arthritis (RA) compared with that of normal control and osteoarthritis (OA), and to examine whether there is a link between HDAC activity and synovial inflammation.
Methods: HDAC activity and histone acetyltransferase (HAT) activity were determined in nuclear extracts of total synovial tissue surgically obtained from normal, OA and RA joints. The level of cytoplasmic tumor necrosis factor a (TNFα) fraction was measured by ELISA. Total RNA of synovial tissue was used for RT-PCR of HDAC1-8. In synovial fibroblasts from RA (RASFs), the effects of TNFα on nuclear HDAC activity and class I HDACs (1, 2, 3, 8) mRNA expressions were examined by quantitative real-time PCR. The protein expression and distribution of class I HDACs were examined by Western blotting.
Results: Nuclear HDAC activity was significantly higher in RA than in OA and normal controls and correlated with the amount of cytoplasmic TNFα. The mRNA expression of HDAC1 in RA synovial tissue was higher than in OA and normal controls, and showed positive correlation with TNFα mRNA expression. The protein level of nuclear HDAC1 was higher in RA synovial tissue compared with OA synovial tissue. Stimulation with TNFα significantly increased the nuclear HDAC activity and HDAC1 mRNA expression at 24 hours and HDAC1 protein expression at 48 hours in RASFs.
Conclusions: Our results showed nuclear HDAC activity and expression of HDAC1 were significantly higher in RA than in OA synovial tissues, and they were upregulated by TNFα stimulation in RASFs. These data might provide important clues for the development of specific small molecule HDAC inhibitors.
Figures
Figure 1
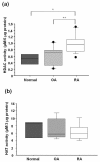
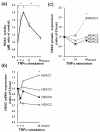
Total nuclear histone deacetylase (HDAC) and histone deacetylase (HAT) activity in synovial tissues. (a) Nuclear HDAC activity in synovial tissues that were obtained from rheumatoid arthritis (RA, n = 14) patients was significantly increased compared to that from normal controls (n = 3) and osteoarthritis (OA, n = 12). In the box (interquartile range, IQR) and whisker (maximum and minimum) plots, the horizontal line inside the box denoted median and the filled circles denote outliers outside IQR ± 1.5 × IQR. * = P < 0.05 versus normal controls, ** = P < 0.05 versus OA. (b) Nuclear HAT activity was measured in synovial tissues that were obtained from normal controls (n = 3), OA (n = 5) and RA patients (n = 5). There was no significant difference between normal controls, OA and RA synovial tissues.
Figure 2
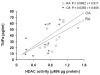
Correlation between the amount of cytoplasmic TNFα and nuclear HDAC activity in synovial tissues. Both cytoplasimc TNFα and nuclear HDAC activity were measured in synovial tissues that were obtained from osteoarthritis (n = 12), and rheumatoid arthritis (n = 12) patients. The cytoplasmic fraction was obtained during the isolation of the nuclear fraction, and analyzed by ELISA.
Figure 3
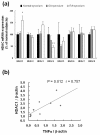
HDAC1-8 mRNA expression and correlation between TNFα mRNA and HDAC1 mRNA expressions in synovial tissues. Total RNA was extracted from total synovial tissues that were obtained from normal controls (n = 3), osteoarthritis (OA, n = 8) and rheumatoid arthritis (RA, n = 9) patients. (a) HDAC1-8 mRNA expression was analyzed by quantitative real-time PCR analysis as described in Materials and Methods. * = P < 0.05 versus OA, ** = P < 0.05 versus normal controls. (b) Correlation between TNFα mRNA expression and HDAC1 mRNA in RA synovial tissues (n = 10).
Figure 4
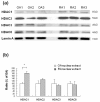
Results of Western blot analysis for nuclear class I HDACs protein expressions in synovial tissues. (a) Class I HDACs (HDAC1, 2, 3, 8) protein expressions in synovial tissues that were obtained from osteoarthritis (OA, n = 3) and rheumatoid arthritis (RA, n = 3) synovial tissues. Lamin A, nuclear membrane protein, served as loading control. (b) Densitometric analysis class I HDACs protein in OA and RA synovial tissue was The data were plotted as means ± SE. * = P < 0.05 versus OA.
Figure 5
Nuclear HDAC activity, class I HDACs mRNA and protein expressions in RASFs after TNFα treatment. RASFs (n = 3) were treated with TNFα (10 ng/ml) and total nuclear protein was extracted at the indicated time points. (a) Nuclear HDAC activity was elevated significantly at 6 h. The data were plotted as means ± SE. * = P < 0.001 versus time 0 h, 24 h. (b) The expression of HDAC1 in RASFs was increased after TNFα treatment, while the expressions of other class I HDACs were not elevated. The increase of mRNA in HDAC1 at 24 h was significantly greater than that in other class I HDACs. For real-time PCR analysis, levels of mRNA were normalized against  actin expression and compared with 0 h. * = P < 0.05 versus HDAC2, 3, 8. (c) Quantitative results of Western blot analysis of nuclear class I HDACs protein expression in RASFs. The band intensity of class I HDACs was measured by Image J software. For analysis, levels of protein expression were normalized by lamin A and compared with 0 h. * = P < 0.05 versus HDAC3 and HDAC8 at 48 h.
actin expression and compared with 0 h. * = P < 0.05 versus HDAC2, 3, 8. (c) Quantitative results of Western blot analysis of nuclear class I HDACs protein expression in RASFs. The band intensity of class I HDACs was measured by Image J software. For analysis, levels of protein expression were normalized by lamin A and compared with 0 h. * = P < 0.05 versus HDAC3 and HDAC8 at 48 h.
Comment in
- Histone deacetylases in RA: epigenetics and epiphenomena.
Grabiec AM, Reedquist KA. Grabiec AM, et al. Arthritis Res Ther. 2010;12(5):142. doi: 10.1186/ar3137. Epub 2010 Oct 11. Arthritis Res Ther. 2010. PMID: 20959025 Free PMC article.
Similar articles
- Histone deacetylase 1 regulates tissue destruction in rheumatoid arthritis.
Hawtree S, Muthana M, Wilkinson JM, Akil M, Wilson AG. Hawtree S, et al. Hum Mol Genet. 2015 Oct 1;24(19):5367-77. doi: 10.1093/hmg/ddv258. Epub 2015 Jul 7. Hum Mol Genet. 2015. PMID: 26152200 - Expression and function of histone deacetylases in rheumatoid arthritis synovial fibroblasts.
Horiuchi M, Morinobu A, Chin T, Sakai Y, Kurosaka M, Kumagai S. Horiuchi M, et al. J Rheumatol. 2009 Aug;36(8):1580-9. doi: 10.3899/jrheum.081115. Epub 2009 Jun 16. J Rheumatol. 2009. PMID: 19531758 - Expression and Functions of Immediate Early Response Gene X-1 (IEX-1) in Rheumatoid Arthritis Synovial Fibroblasts.
Morinobu A, Tanaka S, Nishimura K, Takahashi S, Kageyama G, Miura Y, Kurosaka M, Saegusa J, Kumagai S. Morinobu A, et al. PLoS One. 2016 Oct 13;11(10):e0164350. doi: 10.1371/journal.pone.0164350. eCollection 2016. PLoS One. 2016. PMID: 27736946 Free PMC article. - Efficacy of HDAC inhibitors and epigenetic modulation in the amelioration of synovial inflammation, cellular invasion, and bone erosion in rheumatoid arthritis pathogenesis.
Vijaykrishnaraj M, Patil P, Ghate SD, Bhandary AK, Haridas VM, Shetty P. Vijaykrishnaraj M, et al. Int Immunopharmacol. 2023 Sep;122:110644. doi: 10.1016/j.intimp.2023.110644. Epub 2023 Jul 14. Int Immunopharmacol. 2023. PMID: 37454631 Review. - The roles of HDAC with IMPDH and mTOR with JAK as future targets in the treatment of rheumatoid arthritis with combination therapy.
Mane RR, Kale PP. Mane RR, et al. J Complement Integr Med. 2022 Nov 21;20(4):689-706. doi: 10.1515/jcim-2022-0114. eCollection 2023 Dec 1. J Complement Integr Med. 2022. PMID: 36409592 Review.
Cited by
- Histone deacetylation inhibition in pulmonary hypertension: therapeutic potential of valproic acid and suberoylanilide hydroxamic acid.
Zhao L, Chen CN, Hajji N, Oliver E, Cotroneo E, Wharton J, Wang D, Li M, McKinsey TA, Stenmark KR, Wilkins MR. Zhao L, et al. Circulation. 2012 Jul 24;126(4):455-67. doi: 10.1161/CIRCULATIONAHA.112.103176. Epub 2012 Jun 18. Circulation. 2012. PMID: 22711276 Free PMC article. - The ascent of acetylation in the epigenetics of rheumatoid arthritis.
Grabiec AM, Reedquist KA. Grabiec AM, et al. Nat Rev Rheumatol. 2013 May;9(5):311-8. doi: 10.1038/nrrheum.2013.17. Epub 2013 Feb 26. Nat Rev Rheumatol. 2013. PMID: 23439035 Review. - Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes.
Nygaard G, Firestein GS. Nygaard G, et al. Nat Rev Rheumatol. 2020 Jun;16(6):316-333. doi: 10.1038/s41584-020-0413-5. Epub 2020 May 11. Nat Rev Rheumatol. 2020. PMID: 32393826 Free PMC article. Review. - Role of Histone Deacetylases in Monocyte Function in Health and Chronic Inflammatory Diseases.
Tordera RM, Cortés-Erice M. Tordera RM, et al. Rev Physiol Biochem Pharmacol. 2021;180:1-47. doi: 10.1007/112_2021_59. Rev Physiol Biochem Pharmacol. 2021. PMID: 33974124 Review. - Guizhi-Shaoyao-Zhimu decoction attenuates rheumatoid arthritis partially by reversing inflammation-immune system imbalance.
Guo Q, Mao X, Zhang Y, Meng S, Xi Y, Ding Y, Zhang X, Dai Y, Liu X, Wang C, Li Y, Lin N. Guo Q, et al. J Transl Med. 2016 Jun 8;14(1):165. doi: 10.1186/s12967-016-0921-x. J Transl Med. 2016. PMID: 27277474 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous