Amyloid-beta peptide oligomers disrupt axonal transport through an NMDA receptor-dependent mechanism that is mediated by glycogen synthase kinase 3beta in primary cultured hippocampal neurons - PubMed (original) (raw)
Amyloid-beta peptide oligomers disrupt axonal transport through an NMDA receptor-dependent mechanism that is mediated by glycogen synthase kinase 3beta in primary cultured hippocampal neurons
Helena Decker et al. J Neurosci. 2010.
Abstract
Disruption of axonal transport is a hallmark of several neurodegenerative diseases, including Alzheimer's disease (AD). Even though defective transport is considered an early pathologic event, the mechanisms by which neurodegenerative insults impact transport are poorly understood. We show that soluble oligomers of the amyloid-beta peptide (AbetaOs), increasingly recognized as the proximal neurotoxins in AD pathology, induce disruption of organelle transport in primary hippocampal neurons in culture. Live imaging of fluorescent protein-tagged organelles revealed a marked decrease in axonal trafficking of dense-core vesicles and mitochondria in the presence of 0.5 microm AbetaOs. NMDA receptor (NMDAR) antagonists, including d-AP5, MK-801, and memantine, prevented the disruption of trafficking, thereby identifying signals for AbetaO action at the cell membrane. Significantly, both pharmacological inhibition of glycogen synthase kinase-3beta (GSK-3beta) and transfection of neurons with a kinase-dead form of GSK-3beta prevented the transport defect. Finally, we demonstrate by biochemical and immunocytochemical means that AbetaOs do not affect microtubule stability, indicating that disruption of transport involves a more subtle mechanism than microtubule destabilization, likely the dysregulation of intracellular signaling cascades. Results demonstrate that AbetaOs negatively impact axonal transport by a mechanism that is initiated by NMDARs and mediated by GSK-3beta and establish a new connection between toxic Abeta oligomers and AD pathology.
Figures
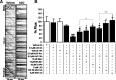
Figure 1.
AβOs block organelle flux in hippocampal neurons. A, Representative kymographs comparing axonal DCV transport in control (vehicle) and AβO-treated neurons (18 h, 0.5 μ
m
AβOs). Organelle flux is markedly reduced in the presence of AβOs (right kymograph). B, Effects of AβOs and other treatments on DCV flux. Disruption of transport can be partially prevented by memantine (Mem), MK-801, lithium, while full protection was obtained with
d
-AP5 and GSK-3β inhibitor VIII (GSK inh.). A minimum of 15 cells from at least 2 different cultures were analyzed per condition; **p < 0.0001 relative to vehicle-treated cultures, *p ranging from 0.007 to 0.05 relative to vehicle-treated cultures in different experimental conditions. #p ranging from 0.0001 to 0.01 relative to AβO-treated cultures in different experimental conditions. +p < 0.05 comparing Mem + AβOs and
d
-AP5 + AβOs; ++p < 0.005 comparing AβOs + LiCl and AβOs + GSK inh. (5 μ
m
). The first 4 bars are not significantly different between them (p > 0.05). Complete statistical evaluation is presented in supplemental Fig. S2, available at
as supplemental material.
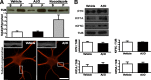
Figure 2.
Expression of kinase-dead GSK-3β (GSK-3β KD) (K85A) prevents AβO-induced transport defects. A, Expression of BDNF-RFP and HA-tagged GSK-3β KD in the same neuron; arrows indicate the axon. B, Expression of GSK-3β KD in neurons prevents AβO-induced transport defects. Conversely, expression of constitutively kinase-active GSK-3β (GSK-3β KA) (S9A) in the absence of AβOs disrupts 50% of DCV transport. Cells were fixed postimaging and stained with anti-HA to confirm the presence of GSK-3β (K85A or S9A). C, Summary of transport data. Vehicle/GSK-3β KD n = 12 kymographs (12 cells, 1930 vesicles); AβO/GSK-3β KD n = 19 kymographs (19 cells, 3468 vesicles); GSK-3β KA n = 13 kymographs (13 cells, 1320 vesicles). *p < 0.05; **p < 0.0001, statistically significant difference compared with vehicle KD; +p < 0.05; ++p < 0.0001, statistically significant difference compared with AβO KD. Scale bar, 25 μm.
Figure 3.
Neuronal cytoskeleton integrity is unaffected by AβO treatment. A, Top, Immunoblots of tubulin from neurons extracted in MT buffer I. The ratio of soluble (S) to polymerized (P) tubulin in vehicle- and AβO-treated cells is unchanged. Bottom, Representative images of tubulin immunocytochemistry in neurons fixed in MT buffer II. B, Motor proteins implicated in the transport of DCVs and/or mitochondria display similar levels between control and AβO-treated neurons. For immunocytochemistry, a minimum of 24 cells per condition from at least 3 different cultures were analyzed; for immunoblots, extracts from three different cultures were analyzed. *p < 0.05; **p < 0.005, statistically significant differences from vehicle-treated neurons (100%). Scale bars, 25 μm.
Similar articles
- Amyloid-β oligomers induce tau-independent disruption of BDNF axonal transport via calcineurin activation in cultured hippocampal neurons.
Ramser EM, Gan KJ, Decker H, Fan EY, Suzuki MM, Ferreira ST, Silverman MA. Ramser EM, et al. Mol Biol Cell. 2013 Aug;24(16):2494-505. doi: 10.1091/mbc.E12-12-0858. Epub 2013 Jun 19. Mol Biol Cell. 2013. PMID: 23783030 Free PMC article. - Modulation of insulin signaling rescues BDNF transport defects independent of tau in amyloid-β oligomer-treated hippocampal neurons.
Takach O, Gill TB, Silverman MA. Takach O, et al. Neurobiol Aging. 2015 Mar;36(3):1378-82. doi: 10.1016/j.neurobiolaging.2014.11.018. Epub 2014 Dec 3. Neurobiol Aging. 2015. PMID: 25543463 - New insights concerning insulin synthesis and its secretion in rat hippocampus and cerebral cortex: amyloid-β1-42-induced reduction of proinsulin level via glycogen synthase kinase-3β.
Nemoto T, Toyoshima-Aoyama F, Yanagita T, Maruta T, Fujita H, Koshida T, Yonaha T, Wada A, Sawaguchi A, Murakami M. Nemoto T, et al. Cell Signal. 2014 Feb;26(2):253-9. doi: 10.1016/j.cellsig.2013.11.017. Epub 2013 Nov 19. Cell Signal. 2014. PMID: 24269535 - GSK3 as a Regulator of Cytoskeleton Architecture: Consequences for Health and Disease.
Hajka D, Budziak B, Pietras Ł, Duda P, McCubrey JA, Gizak A. Hajka D, et al. Cells. 2021 Aug 14;10(8):2092. doi: 10.3390/cells10082092. Cells. 2021. PMID: 34440861 Free PMC article. Review. - Temporal Control of Axonal Transport: The Extreme Case of Organismal Ageing.
Mattedi F, Vagnoni A. Mattedi F, et al. Front Cell Neurosci. 2019 Aug 23;13:393. doi: 10.3389/fncel.2019.00393. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31555095 Free PMC article. Review.
Cited by
- HDAC6 inhibitor blocks amyloid beta-induced impairment of mitochondrial transport in hippocampal neurons.
Kim C, Choi H, Jung ES, Lee W, Oh S, Jeon NL, Mook-Jung I. Kim C, et al. PLoS One. 2012;7(8):e42983. doi: 10.1371/journal.pone.0042983. Epub 2012 Aug 22. PLoS One. 2012. PMID: 22937007 Free PMC article. - [In vitro pathological model of Alzheimer's disease based on neuronal network chip and its real-time dynamic analysis].
Gao F, Gao K, He C, Liu M, Hu Y, Ying K, Wan H, Wang P. Gao F, et al. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2019 Dec 25;36(6):893-901. doi: 10.7507/1001-5515.201902014. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2019. PMID: 31875361 Free PMC article. Retracted. Chinese. - Molecular Mechanisms of Synaptotoxicity and Neuroinflammation in Alzheimer's Disease.
Marttinen M, Takalo M, Natunen T, Wittrahm R, Gabbouj S, Kemppainen S, Leinonen V, Tanila H, Haapasalo A, Hiltunen M. Marttinen M, et al. Front Neurosci. 2018 Dec 14;12:963. doi: 10.3389/fnins.2018.00963. eCollection 2018. Front Neurosci. 2018. PMID: 30618585 Free PMC article. Review. - The Role of Glial Cells and Synapse Loss in Mouse Models of Alzheimer's Disease.
Ziegler-Waldkirch S, Meyer-Luehmann M. Ziegler-Waldkirch S, et al. Front Cell Neurosci. 2018 Dec 11;12:473. doi: 10.3389/fncel.2018.00473. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30618627 Free PMC article. Review. - Tau accumulation causes mitochondrial distribution deficits in neurons in a mouse model of tauopathy and in human Alzheimer's disease brain.
Kopeikina KJ, Carlson GA, Pitstick R, Ludvigson AE, Peters A, Luebke JI, Koffie RM, Frosch MP, Hyman BT, Spires-Jones TL. Kopeikina KJ, et al. Am J Pathol. 2011 Oct;179(4):2071-82. doi: 10.1016/j.ajpath.2011.07.004. Epub 2011 Aug 18. Am J Pathol. 2011. PMID: 21854751 Free PMC article.
References
- Blurton-Jones M, Laferla FM. Pathways by which Abeta facilitates tau pathology. Curr Alzheimer Res. 2006;3:437–448. - PubMed
- Brady ST, Lasek RJ, Allen RD, Yin HL, Stossel TP. Gelsolin inhibition of fast axonal transport indicates a requirement for actin microfilaments. Nature. 1984;310:56–58. - PubMed
- De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Abeta oligomers induce neuronal oxidative stress through an N-methyl-d-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources